1098209
USP
Cefuroxime sodium
United States Pharmacopeia (USP) Reference Standard
Synonim(y):
Cefuroxime sodium salt
About This Item
Polecane produkty
klasa czystości
pharmaceutical primary standard
rodzina API
cefuroxime
producent / nazwa handlowa
USP
Zastosowanie
pharmaceutical (small molecule)
format
neat
temp. przechowywania
−20°C
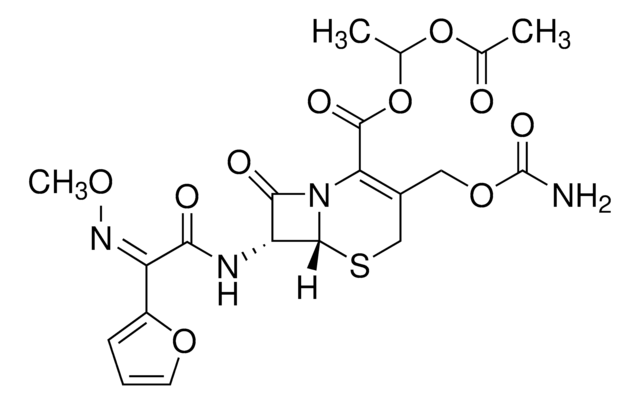
ciąg SMILES
[Na+].CO\N=C(/C(=O)N[C@H]1[C@H]2SCC(COC(N)=O)=C(N2C1=O)C([O-])=O)c3ccco3
InChI
1S/C16H16N4O8S.Na/c1-26-19-9(8-3-2-4-27-8)12(21)18-10-13(22)20-11(15(23)24)7(5-28-16(17)25)6-29-14(10)20;/h2-4,10,14H,5-6H2,1H3,(H2,17,25)(H,18,21)(H,23,24);/q;+1/p-1/b19-9-;/t10-,14-;/m1./s1
Klucz InChI
URDOHUPGIOGTKV-JTBFTWTJSA-M
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Komentarz do analizy
Inne uwagi
produkt powiązany
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Skin Sens. 1
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej