Wszystkie zdjęcia(1)
Kluczowe dokumenty
80614
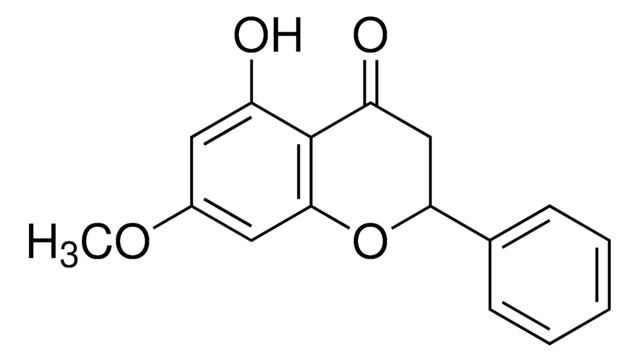
Pinostrobin
≥99.0% (TLC)
Synonim(y):
(S)-2,3-Dihydro-5-hydroxy-7-methoxy-2-phenyl-4H-1-benzopyran-4-one
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór empiryczny (zapis Hilla):
C16H14O4
Numer CAS:
Masa cząsteczkowa:
270.28
Beilstein:
270230
Numer WE:
Numer MDL:
Kod UNSPSC:
12352200
Identyfikator substancji w PubChem:
Polecane produkty
Próba
≥99.0% (TLC)
ciąg SMILES
COc1cc(O)c2C(=O)C[C@H](Oc2c1)c3ccccc3
InChI
1S/C16H14O4/c1-19-11-7-12(17)16-13(18)9-14(20-15(16)8-11)10-5-3-2-4-6-10/h2-8,14,17H,9H2,1H3/t14-/m0/s1
Klucz InChI
ORJDDOBAOGKRJV-AWEZNQCLSA-N
Działania biochem./fizjol.
Elicits intense apoptotic response from cultured leukemia cells in vitro. Strongly inhibits the Ca2+ signals involved in the control of G2/M phase cell cycle progression in Saccharomyces cerevisiae. Shows potent antiviral effect against herpes simplex virus-1.
Opakowanie
Bottomless glass bottle. Contents are inside inserted fused cone.
Ta strona może zawierać tekst przetłumaczony maszynowo.
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Lot/Batch Number
Przepraszamy, ale COA dla tego produktu nie jest aktualnie dostępny online.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Hadi Poerwono et al.
Bioorganic & medicinal chemistry letters, 20(7), 2086-2089 (2010-03-12)
Pinostrobin (5-hydroxy-7-methoxyflavanone) obtained in relatively large amounts from fingerroot (Boesenbergia pandurata) was converted to its C-6 and C-8 prenylated derivatives. The Mitsunobu reaction, europium(III)-catalyzed Claisen-Cope rearrangement, and Claisen reaction coupled with cross-metathesis were used as the key steps. Using a
J C Le Bail et al.
Cancer letters, 156(1), 37-44 (2000-06-07)
The interaction between the estrogen receptor and 5-hydroxy-7-methoxyflavanone (pinostrobin) was studied in the presence or absence of estradiol or dehydroepiandrosterone sulfate (DHEAS), respectively, using a stably transfected human breast cancer cell line (MVLN). We also evaluated its action on the
Nwet Nwet Win et al.
Journal of natural products, 70(10), 1582-1587 (2007-09-28)
The chloroform extract of rhizomes of Boesenbergia pandurata demonstrated marked preferential cytotoxicity against human pancreatic PANC-1 cancer cells in nutrient-deprived medium. Bioactivity-directed investigation of this extract yielded four new secondary metabolites, geranyl-2,4-dihydroxy-6-phenethylbenzoate ( 1), 2',4'-dihydroxy-3'-(1''-geranyl)-6'-methoxychalcone ( 2), (1' R,2' S,6'
Chavi Yenjai et al.
Bioorganic & medicinal chemistry letters, 20(9), 2821-2823 (2010-04-07)
Flavones 1-4 isolated from Kaempferia parviflora were used for structural modification. Sixteen flavonoid derivatives, including four new derivatives, were synthesized and evaluated for cytotoxicity against KB and NCI-H187 cell lines. Flavanones 2a-4a demonstrated higher cytotoxic activity than the parent compounds.
Tan Siew Kiat et al.
Bioorganic & medicinal chemistry letters, 16(12), 3337-3340 (2006-04-20)
Boesenbergia rotunda (L.) cyclohexenyl chalcone derivatives, 4-hydroxypanduratin A and panduratin A, showed good competitive inhibitory activities towards dengue 2 virus NS3 protease with the Ki values of 21 and 25 microM, respectively, whilst those of pinostrobin and cardamonin were observed
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej