U5252
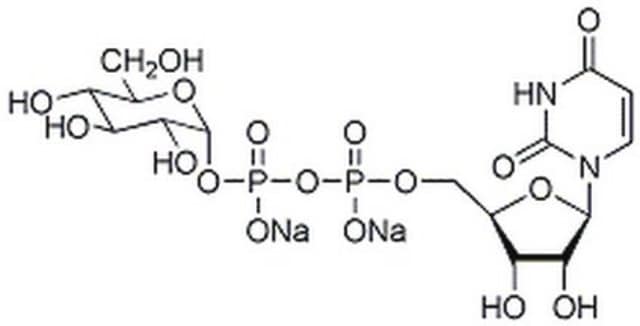
Uridine 5′-diphospho-N-acetylgalactosamine disodium salt
≥97%
Synonym(s):
(UDP)-GalNAc, UDP-GalNAc, UDP-N-acetylgalactosamine, Uridine[5′]diphospho[1](2-acetamino-2-deoxy-α-D-galactopyranose) disodium salt
About This Item
Recommended Products
biological source
synthetic (organic)
Assay
≥97%
form
powder
storage temp.
−20°C
SMILES string
[Na+].[Na+].CC(=O)N[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1OP([O-])(=O)OP([O-])(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)N3C=CC(=O)NC3=O
InChI
1S/C17H27N3O17P2.2Na/c1-6(22)18-10-13(26)11(24)7(4-21)35-16(10)36-39(31,32)37-38(29,30)33-5-8-12(25)14(27)15(34-8)20-3-2-9(23)19-17(20)28;;/h2-3,7-8,10-16,21,24-27H,4-5H2,1H3,(H,18,22)(H,29,30)(H,31,32)(H,19,23,28);;/q;2*+1/p-2/t7-,8-,10-,11+,12-,13-,14-,15?,16?;;/m1../s1
InChI key
HXWKMJZFIJNGES-QCVFHWOISA-L
Looking for similar products? Visit Product Comparison Guide
General description
Application
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
LC-MS/MS method quantifies similar polar nucleotide activated sugars using Supel™ Carbon LC column for simultaneous analysis.
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
Enzymatic glycosyltransferase specificity challenges the one enzyme-one linkage concept.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service