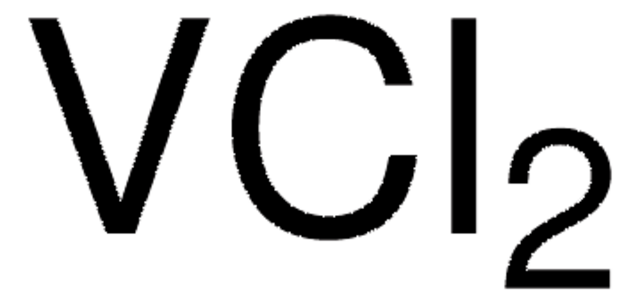1.12393
Vanadium(III) chloride
99+
Synonym(s):
Vanadium(III) chloride, Vanadium trichloride
About This Item
Recommended Products
Quality Level
form
solid
potency
350 mg/kg LD50, oral (Rat)
density
3.0 g/cm3 at 20 °C
bulk density
1000 kg/m3
storage temp.
no temp limit
InChI
1S/3ClH.V/h3*1H;/q;;;+3/p-3
InChI key
HQYCOEXWFMFWLR-UHFFFAOYSA-K
Related Categories
Application
- Dihydropyrimidinone derivatives via a three-component Biginelli condensation reaction between aldehyde, β-keto ester, and urea (thiourea).
- Coumarin derivatives via Pechmann condensation reaction of various phenols and β-keto esters.
- β-amino alcohols by deprotection of epoxides with aromatic amines.
- Diol derivatives by chemoselective cleavage of acetonides in methanol.
VCl3 can also be used as a reducing agent to determine nitrates and nitrites in water samples.
Analysis Note
Identity (ICP-OES): passes test
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
8B - Non-combustible, corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service