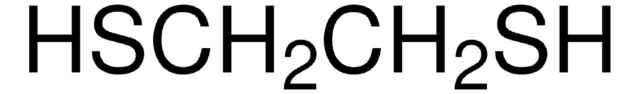All Photos(1)
About This Item
Linear Formula:
CH3SC6H9(=O)
CAS Number:
Molecular Weight:
144.23
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.508 (lit.)
bp
45 °C/10.1 mmHg (lit.)
density
1.069 g/mL at 25 °C (lit.)
SMILES string
CSC1CCCCC1=O
InChI
1S/C7H12OS/c1-9-7-5-3-2-4-6(7)8/h7H,2-5H2,1H3
InChI key
QFABNUVNDKOIEH-UHFFFAOYSA-N
General description
2-(Methylthio)cyclohexanone is a 2-substituted cyclohexanone. The proportion of axial and equatorial conformation has been measured in chloroform by the Eliel method. It suggests that the in chloroform steric effect dominates over polar effect in contributing to the conformational preference. The stereoselectivity of the reduction of 2-(methylthio)cyclohexanone using different hydrides has been studied.
Application
2-(Methylthio)cyclohexanone may be used as a ketone substrate for the synthesis of cyclic nitrones, by reacting with aspergillusol A.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
192.2 °F - closed cup
Flash Point(C)
89 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Axial/equatorial proportions for 2-substituted cyclohexanones.
Basso EA, et al.
The Journal of Organic Chemistry, 58(27), 7865-7869 (1993)
Nitrone formation in phosphate buffer and aqueous solutions: novel chemistry inspired by a natural product.
Pansanit A, et al.
Tetrahedron Letters, 53(16), 2129-2131 (2012)
Studies on the stereoselectivity of hydride reductions on 2-(methylthio)-and 2-(methylsulfonyl) cyclohexanones.
Carmen CM, et al.
The Journal of Organic Chemistry, 52(16), 3619-3625 (1987)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service