421235
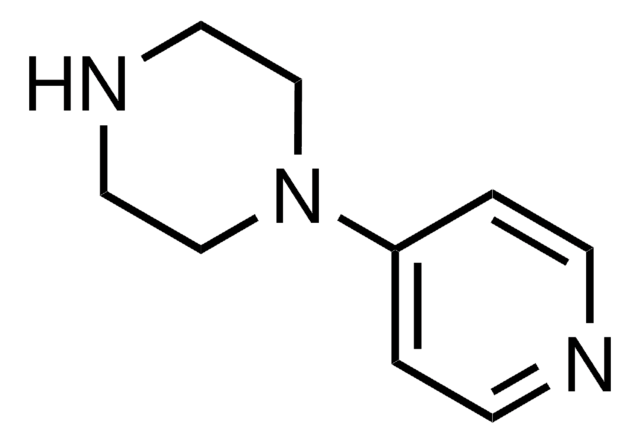
1-(2-Pyrimidyl)piperazine
98%
Synonym(s):
2-(1-Piperazinyl)pyrimidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H12N4
Molecular Weight:
164.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.587 (lit.)
bp
277 °C (lit.)
density
1.158 g/mL at 25 °C (lit.)
SMILES string
C1CN(CCN1)c2ncccn2
InChI
1S/C8H12N4/c1-2-10-8(11-3-1)12-6-4-9-5-7-12/h1-3,9H,4-7H2
InChI key
MRBFGEHILMYPTF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-(2-Pyrimidyl)piperazine is a piperazine-based derivative. It is a metabolite of buspirone.
Application
1-(2-Pyrimidyl)piperazine may be used for the following studies:
- As derivatization reagent for the carboxyl groups on peptides.
- Carboxy group derivatization during the spectrophotometric analysis of phosphopeptides.
- Starting reagent for the synthesis of 3-{(4-(pyrimidin-2-yl)piperazin-1-yl)methyl}-1H-pyrrolo[2,3-b]pyridine.
- Synthesis of 3-phenyl-6-(4-(pyrimidin-2-yl)piperazin-1-yl)pyridazin-4-ol.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
>230.0 °F
Flash Point(C)
> 110 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Hascoët et al.
Pharmacology, biochemistry, and behavior, 67(1), 45-53 (2000-12-13)
Although numerous animal procedures have been employed in the study of antidepressants (ADs) in anxiety, the results following acute administration remain highly variable. The present study investigated the effect of the SSRI paroxetine (4, 8, and 16 mg/kg, IP) in
Xiaoqiang Qiao et al.
Rapid communications in mass spectrometry : RCM, 25(5), 639-646 (2011-02-04)
Piperazine-based derivatives, including 1-(2-pyridyl)piperazine (2-PP), 1-(2-pyrimidyl)piperazine (2-PMP), 1-(4-pyridyl)piperazine (4-PP), and 1-(1-methyl-4-piperidinyl)piperazine (M-PP), were used for the derivatization of carboxyl groups on peptides with 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and 1-hydroxy-7-azabenzotriazole (HOAt) as coupling reagents, and trifluoroacetic acid (TFA) as activator. Taking synthetic
A J Gower et al.
European journal of pharmacology, 155(1-2), 129-137 (1988-10-11)
The anxiolytic effects of buspirone, its metabolite, 1-(2-pyrimidyl)piperazine (1-PP) and several alpha 2-adrenoceptor antagonists have been compared in an anticonflict (shock-induced suppression of drinking) paradigm in rats. Idazoxan, WY 26392 and yohimbine had anticonflict effects comparable to those of buspirone
A H Vaidya et al.
Methods and findings in experimental and clinical pharmacology, 27(4), 245-255 (2005-08-06)
Most studies concerning the effects of oral buspirone in the rat elevated plus-maze (EPM) test, spontaneous motor activity (SMA) test, and Vogel conflict (VC) test have used Sprague-Dawley or Wistar rats. Although it has been documented that the behavior of
I Mahmood et al.
Clinical pharmacokinetics, 36(4), 277-287 (1999-05-13)
Buspirone is an anxiolytic drug given at a dosage of 15 mg/day. The mechanism of action of the drug is not well characterised, but it may exert its effect by acting on the dopaminergic system in the central nervous system
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service