All Photos(3)
About This Item
Linear Formula:
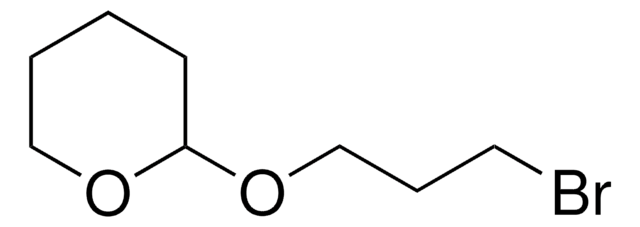
C6H5CH2O(CH2)3Br
CAS Number:
Molecular Weight:
229.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.531 (lit.)
bp
130-132 °C/8 mmHg (lit.)
density
1.298 g/mL at 25 °C (lit.)
SMILES string
BrCCCOCc1ccccc1
InChI
1S/C10H13BrO/c11-7-4-8-12-9-10-5-2-1-3-6-10/h1-3,5-6H,4,7-9H2
InChI key
PSUXTZLDBVEZTD-UHFFFAOYSA-N
Related Categories
General description
Benzyl 3-bromopropyl ether is an ether.
Application
Benzyl 3-bromopropyl ether may be used in the following studies:
- Preparation of (2S,3S)-1-[(triisopropylsilyl)oxy]-7-(benzyloxy)-2,3-(isopropylidenedioxy)-4(Z)-heptene.
- Preparation of 5-(3-Benzyloxypropoxy)psoralen (PAP-7).
- Total synthesis of zincophorin and (+)-anatoxin-a.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Magali Defosseux et al.
The Journal of organic chemistry, 69(14), 4626-4647 (2004-07-03)
A total synthesis of the naturally occurring ionophore zincophorin has been realized. The route features an intramolecular oxymercuration of a cyclopropanemethanol and a Carroll-Claisen rearrangement for the respective elaboration of the C1-C12 and C13-C25 subunits, which have been assembled by
Alexander Schmitz et al.
Molecular pharmacology, 68(5), 1254-1270 (2005-08-16)
The lymphocyte K+ channel Kv1.3 constitutes an attractive pharmacological target for the selective suppression of terminally differentiated effector memory T (TEM) cells in T cell-mediated autoimmune diseases, such as multiple sclerosis and type 1 diabetes. Unfortunately, none of the existing
Tetrahedron Letters, 45, 4397-4399 (2004)
Synthesis of natural and modified trapoxins, useful reagents for exploring histone deacetylase function.
Taunton J, et al.
Journal of the American Chemical Society, 118(43), 10412-10422 (1996)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service