337757
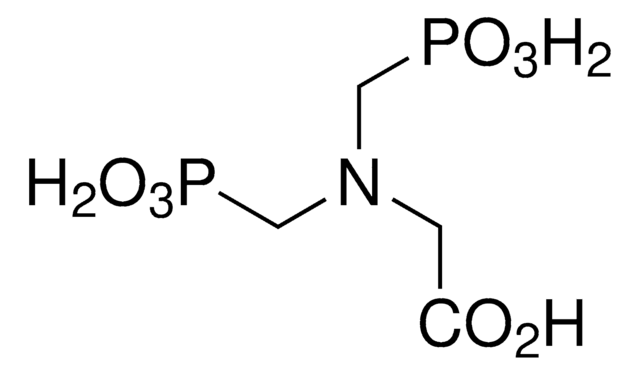
N-(Phosphonomethyl)glycine
96%, for peptide synthesis
Synonym(s):
Glyphosate
About This Item
Recommended Products
product name
N-(Phosphonomethyl)glycine, 96%
Assay
96%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
mp
230 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
OC(=O)CNCP(O)(O)=O
InChI
1S/C3H8NO5P/c5-3(6)1-4-2-10(7,8)9/h4H,1-2H2,(H,5,6)(H2,7,8,9)
InChI key
XDDAORKBJWWYJS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Water Pollution Analysis: Campanale et al. assessed glyphosate and AMPA pesticides in river waters and sediments, providing crucial data on the environmental distribution and persistence of N-(Phosphonomethyl)glycine derivatives. This research supports efforts to monitor and regulate environmental pollutants effectively (Campanale et al., 2024).
- Public Health Studies: Urinary biomonitoring of glyphosate exposure was conducted among farmers, utilizing N-(Phosphonomethyl)glycine as a marker. This study contributes to our understanding of occupational exposure risks and supports the development of health safety guidelines (Chang et al., 2024).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Aquatic Chronic 2 - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
EPA Method 547 outlines the analysis of glyphosate in drinking water by direct aqueous injection HPLC, post column derivatization, and fluorescence detection
LC/MS Analysis of Glyphosate and Metabolites on apHera™ NH2, 2 mm I.D. Column
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service