293474
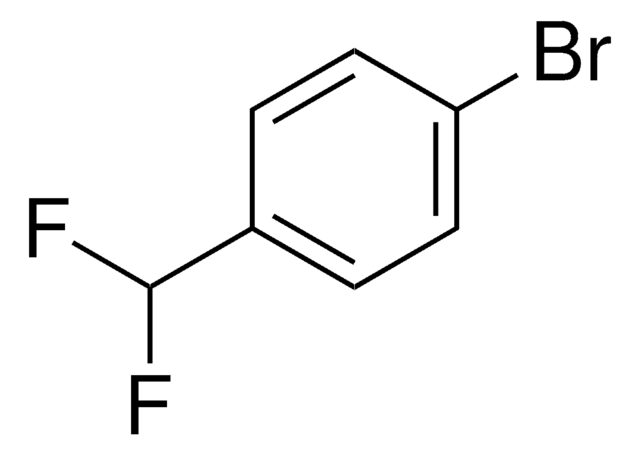
4-Bromo-2-fluoroanisole
97%
Synonym(s):
4-Bromo-2-fluoro-1-methoxybenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H3(F)OCH3
CAS Number:
Molecular Weight:
205.02
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.545 (lit.)
bp
84 °C/7 mmHg (lit.)
density
1.59 g/mL at 25 °C (lit.)
functional group
bromo
fluoro
SMILES string
COc1ccc(Br)cc1F
InChI
1S/C7H6BrFO/c1-10-7-3-2-5(8)4-6(7)9/h2-4H,1H3
InChI key
DWNXGZBXFDNKOR-UHFFFAOYSA-N
General description
The IR and FT-IR spectra of 4-bromo-2-fluoroanisole have been investigated.
Application
4-Bromo-2-fluoroanisole has been used in the synthesis of:
- 1,4-bis[(3′-fluoro-4′-n alkoxyphenyl)ethynyl]benzenes with n=1-12
- 7-fluoro-6-methoxy-1-methyl-2-naphthaldehyde
- 5,7-difluoro-6-methoxy-1-methyl-2-naphthaldehyde
- liquid crystals with terminal difluoromethoxy group and backbone of phenylbicyclohexane
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
208.4 °F - closed cup
Flash Point(C)
98 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Design of molecular architectures for polymeric mesophase formation.
Pugh C, et al.
Macromolecular Symposia, 98(1) (1995)
Vibrational spectra, first hyperpolarizability, HOMO-LUMO analysis of 4-bromo 2-fluro anisole.
Arivazhagan M, et al.
Indian Journal of Pure and Applied Physics, 50(5), 299-307 (2012)
Rolf W Hartmann et al.
Journal of enzyme inhibition and medicinal chemistry, 19(2), 145-155 (2004-09-29)
Regioselectively fluorinated 1-(naphth-2-ylmethyl)imidazoles 1a-h have been synthesized starting from the corresponding (naphth-2-yl)methanols (2). 2a-d have been obtained by LiAlH4-promoted reduction of fluorinated 1-methyl-2-naphthaldehydes. The latter were easily prepared in fairly good overall yields by ceric ammonium nitrate (CAN)-promoted oxidative addition
Syntheses of Phenylbicyclohexane Liquid Crystals with Terminal Difluoromethoxy Group [J].
Li J, et al.
Fine Chemicals / ????, 12, 004-004 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service