All Photos(3)
About This Item
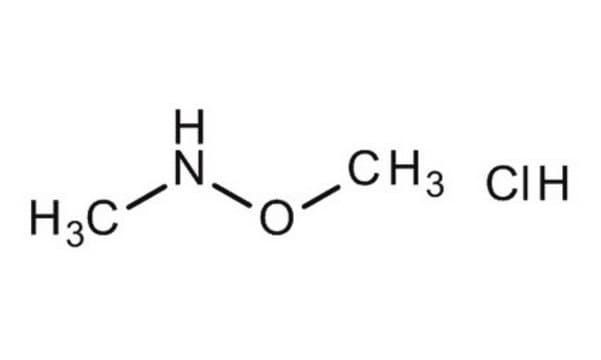
Linear Formula:
(CH3)2NOH · HCl
CAS Number:
Molecular Weight:
97.54
Beilstein:
3905683
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
107-109 °C (lit.)
SMILES string
Cl[H].CN(C)O
InChI
1S/C2H7NO.ClH/c1-3(2)4;/h4H,1-2H3;1H
InChI key
HWWVAHCWJLGKLW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N,N-Dimethylhydroxylamine hydrochloride was used in the synthesis of 4,4-dimethyl-2,5,5-triphenyl-l.3-dioxa-4-azonia-2-bora-5-boratacyclopentane. It was also used as a polymer-chain terminator.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Structural studies of organoboron compounds. XVI. Preparation and crystal and molecular structures of 4, 4-dimethyl-2, 5, 5-triphenyl-1, 3-dioxa-4-azonia-2-bora-5-boratacyclopentane and 4, 4, 5, 5-tetramethyl-2, 2-diphenyl-1, 3-dioxa-4-azonia-2-boratacyclopentane.
Canadian Journal of Chemistry, 62(5), 838-844 (1984)
Hana Popelkova et al.
Photosynthesis research, 110(2), 111-121 (2011-11-02)
The photosystem II (PSII) manganese-stabilizing protein (PsbO) is known to be the essential PSII extrinsic subunit for stabilization and retention of the Mn and Cl(-) cofactors in the oxygen evolving complex (OEC) of PSII, but its function relative to Ca(2+)
J Taira et al.
Biochimica et biophysica acta, 1336(3), 502-508 (1997-11-21)
Hydroxylamine (HA), which is a natural product of mammalian cells, has been shown to possess vasodilatory properties in several model systems. In this study, HA and methyl-substituted hydroxylamines, N-methylhydroxylamine (NMHA) and N,N-dimethylhydroxylamine (NDMHA), have been tested for their ability to
A A Spooren et al.
Archives of toxicology, 71(5), 299-305 (1997-01-01)
Hydroxylamine (HYAM, HONH2) and some of its derivatives are known to cause erythrotoxic effects both in vitro and in vivo. Previous studies have shown that the primary in vitro effect of HYAM and O-ethyl hydroxylamine (OEH) is methaemoglobin formation, leading
K Stolze et al.
Free radical research communications, 8(2), 123-131 (1990-01-01)
Nitroxide radicals have been detected in the methemoglobin formation reaction between oxyhemoglobin and the substituted hydroxylamine compounds, N-methylhydroxylamine and N,N-dimethylhydroxylamine, by ESR spectroscopy. The stability of these nitroxide radicals was considerably higher than that of the NH2O. radical derived from
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service