163392
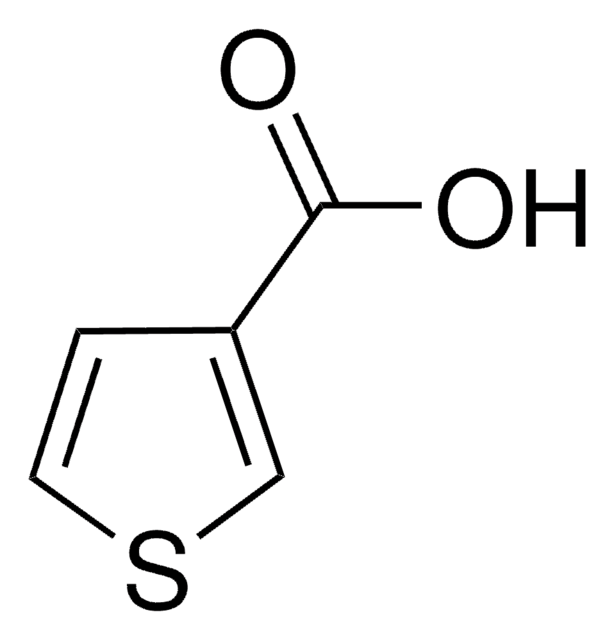
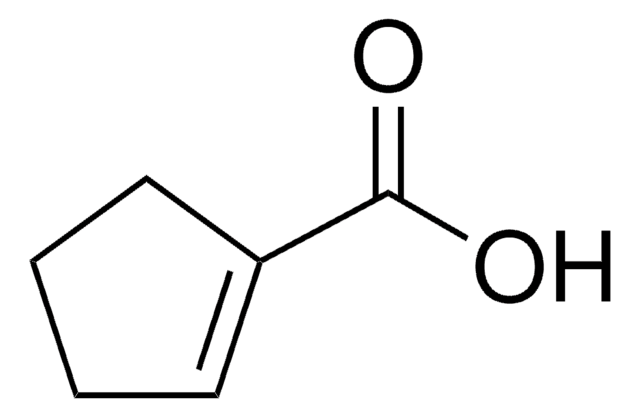
3-Furoic acid
98%
Synonym(s):
Furan-3-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H4O3
CAS Number:
Molecular Weight:
112.08
Beilstein:
108638
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
120-122 °C (lit.)
SMILES string
OC(=O)c1ccoc1
InChI
1S/C5H4O3/c6-5(7)4-1-2-8-3-4/h1-3H,(H,6,7)
InChI key
IHCCAYCGZOLTEU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Furoic acid can be used as a reactant to synthesize:
- Furan-2,5- and furan-2,4-dicarboxylic acid under solvent-free conditions via disproportionation reaction.
- (±)-Hyperolactone A by reacting with 2-methylbutanal.
- Furo[2,3-b]pyridin-4-one-5-carboxylate ester derivatives as potential non-nucleoside inhibitors of human herpesvirus polymerases.
Biochem/physiol Actions
3-Furoic acid exhibits hypolipidemic activity in rodents. It lowers serum cholesterol and serum triglyceride levels in mice and rats.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of 4-oxo-4, 7-dihydrofuro [2, 3-b] pyridine-5-carboxamides with broad-spectrum human herpesvirus polymerase inhibition
Schnute ME, et al.
Bioorganic & Medicinal Chemistry Letters, 18(14), 3856-3859 (2008)
First total synthesis of (?)-hyperolactone A
Ichinari D, et al.
Chemical Communications (Cambridge, England), (18), 1743-1744 (1997)
Concurrent formation of furan-2, 5-and furan-2, 4-dicarboxylic acid: unexpected aspects of the Henkel reaction.
Thiyagarajan S, et al.
Royal Society of Chemistry Advances, 3(36), 15678-15686 (2013)
I H Hall et al.
Pharmaceutical research, 2(5), 233-238 (1985-09-01)
2-Furoic acid, 3-furoic acid, 3,4-furan dicarboxylic acid and furyl-acrylic acid were evaluated for hypolipidemic activity in mice and rats. 2-Furoic acid was the most potent agent of the four tested, lowering serum cholesterol levels 41 % and serum triglyceride levels
Concurrent formation of furan-2, 5-and furan-2, 4-dicarboxylic acid: unexpected aspects of the Henkel reaction
Thiyagarajan S, et al.
Royal Society of Chemistry Advances, 3(36), 15678-15686 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service