G3002
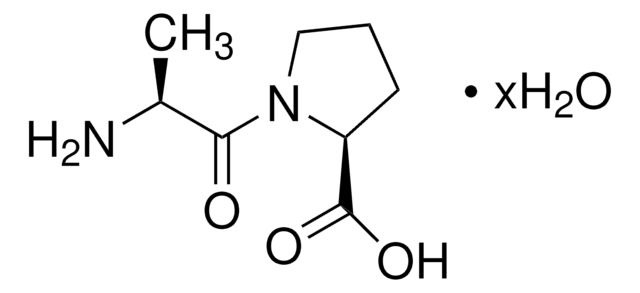
Gly-Pro
≥98%, suitable for ligand binding assays
Synonym(s):
glycyl-L-proline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
product name
Gly-Pro,
Assay
≥98%
form
solid
technique(s)
ligand binding assay: suitable
color
white
storage temp.
−20°C
SMILES string
NCC(N1[C@H](C(O)=O)CCC1)=O
InChI
1S/C7H12N2O3/c8-4-6(10)9-3-1-2-5(9)7(11)12/h5H,1-4,8H2,(H,11,12)
InChI key
KZNQNBZMBZJQJO-UHFFFAOYSA-N
Application
- Diagnostic efficacy of [(68)Ga]Ga-DOTA-GPFAPI-04 in patients with solid tumors in a head-to-head comparison with [(18)F]F-FDG: results from a prospective clinical study.: This study explores the use of Gly-Pro in peptide imaging agents, specifically [(68)Ga]Ga-DOTA-GPFAPI-04, demonstrating its superior diagnostic performance in detecting solid tumors compared to traditional imaging modalities, offering new avenues for cancer diagnosis and monitoring (Yuan et al., 2024).
- Understanding the Transepithelial Transport and Transbilayer Diffusion of the Antihypertensive Peptide Asn-Cys-Trp: Insights from Caco-2 Cell Monolayers and the DPPC Model Membrane.: This research demonstrates the utility of Gly-Pro in studying the absorption and metabolic stability of antihypertensive peptides, providing key insights into their pharmacokinetic behaviors and enhancing drug delivery strategies (Wu et al., 2024).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Poonam Kalhotra et al.
Biomolecules, 10(2) (2020-02-23)
Diabetes mellitus is a severe health problem in Mexico, and its prevalence is increasing exponentially every year. Recently, DPP-4 (dipeptidyl peptidase-4) inhibitors have become attractive oral anti-hyperglycemic agents to reduce the pathology of diabetes. Gliptin's family, such as sitagliptin, vildagliptin
E Berardesca et al.
The British journal of dermatology, 126(2), 193-195 (1992-02-01)
A case is reported of a 15-year-old boy with prolidase deficiency and marked urinary excretion of the iminodipeptide gly-pro. Prolidase activity of erythrocytes against substrate glycyl-proline was deficient, but after blood transfusions this was increased to 15.7% of donor activity
Mitsuru Tanaka et al.
Scientific reports, 9(1), 5769-5769 (2019-04-10)
Apart from nutrients required for the brain, there has been no report that naturally occurring peptides can cross the blood-brain barrier (BBB). The aim of this study was to identify the BBB-transportable peptides using in situ mouse perfusion experiments. Based
Emily Mason et al.
Foods (Basel, Switzerland), 9(7) (2020-07-18)
The bioactive properties and health-promoting effects of two novel yellow (C09052, C05041) and two brown (Calvi, Bastia) hairless canary seed (Phalaris canariensis L.) cultivars were investigated in comparison to two common cereal grains (wheat and oat). The cereal flours were
C Méthivier et al.
Faraday discussions, 204, 69-81 (2017-08-03)
Adsorption of the Glycine-Proline (Gly-Pro) dipeptide has been investigated using surface science complementary techniques on Au(110) and Ag(110), showing some interesting differences both in the chemical form and surface organization of the adsorbed peptide. On Au(110), Gly-Pro mainly adsorbs in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service