912972
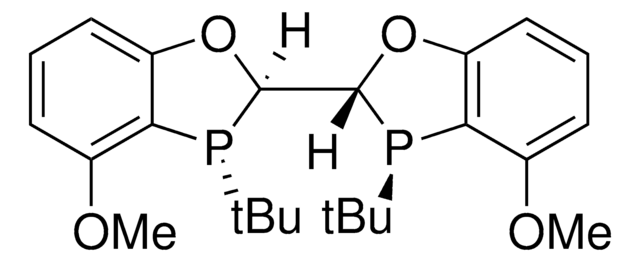
(2S,2′S,3S,3′S)-MeO-BIBOP
≥97%
Synonym(s):
(2S,2′S,3S,3′S)-3,3′-di-tert-butyl-4,4′-dimethoxy-2,2′,3,3′-tetrahydro-2,2′-bibenzo[d][1,3]oxaphosphole
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C24H32O4P2
CAS Number:
Molecular Weight:
446.46
UNSPSC Code:
12352200
Recommended Products
Assay
≥97%
form
powder
optical purity
ee: ≥99% (HPLC)
reaction suitability
reagent type: ligand
functional group
phosphine
Application
(2S,2′S,3S,3′S)-MeO-BIBOP is a P-chiral biphosphorus ligand used in a variety of asymmetric transition metal-catalyzed transformations including hydrogenations, propargylations, reductions, and hydroformylations.
Product can be used with our benchtop hydrogen generator, H-Genie Lite (Z744083)
Product can be used with our benchtop hydrogen generator, H-Genie Lite (Z744083)
Legal Information
Sold in collaboration with Zejun Pharmaceuticals
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Wenjun Tang et al.
Organic letters, 12(1), 176-179 (2009-12-03)
A series of novel, efficient, air-stable, and tunable chiral bisdihydrobenzooxaphosphole ligands (BIBOPs) were developed for rhodium-catalyzed hydrogenations of various functionalized olefins such as alpha-arylenamides, alpha-(acylamino)acrylic acid derivatives, beta-(acylamino)acrylates, and dimethyl itaconate with excellent enantioselectivities (up to 99% ee) and reactivities
Renchang Tan et al.
Organic letters, 18(14), 3346-3349 (2016-06-23)
Air-stable and tunable chiral bisdihydrobenzooxaphosphole ligands (BIBOPs) were employed in rhodium-catalyzed asymmetric hydroformylation of various terminal olefins with excellent conversions (>99%), moderate-to-excellent enantioselectivities (up to 95:5 er), and branched to linear ratios (b:l) of up to 400.
Chengxi Li et al.
Angewandte Chemie (International ed. in English), 58(38), 13573-13583 (2019-07-26)
We herein report the development of a conformationally defined, electron-rich, C2 -symmetric, P-chiral bisphosphorus ligand, ArcPhos, by taking advantage of stereoelectronic effects in ligand design. With the Rh-ArcPhos catalyst, excellent enantioselectivities and unprecedentedly high turnovers (TON up to 10 000) were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service