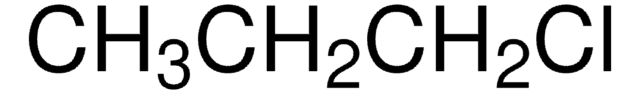338303
Chloroethane solution
2.0 M in tert-butyl methyl ether, anhydrous
Synonym(s):
Ethyl chloride
About This Item
Recommended Products
grade
anhydrous
vapor density
2.22 (vs air)
vapor pressure
20.03 psi ( 55 °C)
5.98 psi ( 20 °C)
form
liquid
autoignition temp.
966 °F
concentration
2.0 M in tert-butyl methyl ether
impurities
<0.005% water
density
0.775 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
CCCl
InChI
1S/C2H5Cl/c1-2-3/h2H2,1H3
InChI key
HRYZWHHZPQKTII-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Green solvent in biomaterial extraction: Research highlights the use of Chloroethane as an effective solvent for extracting lignin-containing nanocellulose fibrils from date palm waste, emphasizing its potential in enhancing the sustainability of biomaterial processing (Raza et al., 2024).
Packaging
Compatible with the following:
- Aldrich® Sure/Pac™ station for liquefied gases Z566446
- PTFE Sealing tape Z104388 or Z221880
- Straight septum-inlet adapter Z118141 with septa Z565687 or Z565695
Legal Information
also commonly purchased with this product
recommended
septum inlet adapter
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Flam. Liq. 2 - Repr. 1B - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
-29.2 °F - closed cup
Flash Point(C)
-34 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service