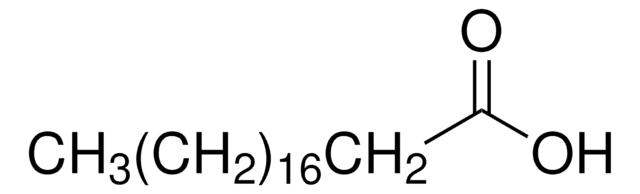A3352
Acyl-coenzyme A Synthetase from Pseudomonas sp.
≥2 units/mg protein
Synonym(s):
Acid: CoA ligase (AMP-forming)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
Looking for similar products? Visit Product Comparison Guide
General description
Acyl-coenzyme A synthetase belongs to adenylate-forming enzymes superfamily. It has a conserved adenosine triphosphate/adenosine monophosphate (ATP/AMP) binding motif.
Application
Acyl-coenzyme A (coA) synthetase from Pseudomonas sp. has been used:
- as ligase in the synthesis of mevalonate derivatives of adenosine triphosphate (ATP)
- in in vitro fatty acylation assays
- in the synthesis of (14C)Vernoloyl-CoA (Va-CoA),(3) bisphosphonates derivatives of ATP(4) and (3H)Palmitate-CoA
Acyl-coenzyme A Synthetase may be used to study fatty acid metabolism and lipid metabolism. It has been used to study its interaction with fatty acid transport proteins, which has been found to be involved in the efficient cellular uptake of long-chain fatty acids in adipocytes .
Biochem/physiol Actions
Acyl coenzyme A synthetase proteins are involved in regulating and facilitating long-chain fatty acid transport in mammalian cells .
Acyl-coenzyme A (CoA) synthetase (ACS) enzyme catalyzes a two-step thioesterification reaction involving the conversion of free fatty acids (FAs) to CoA esters. The substrate for ACS varies from two carbon to 26 carbon FAs. It is involved in the activation of FAs for lipid metabolism and enables FA to participate in various cellular metabolic pathways.
Unit Definition
One unit will form 1.0 μmole of AMP and oleoyl coenzyme A from ATP and oleate at pH 8.1 at 25 °C in the presence of Coenzyme A.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Ruść et al.
Meat science, 89(4), 440-443 (2011-06-10)
The aim of the study was to check whether different genotypes at acyl-CoA synthetase (ACSL4 locus, SNP G2645A) are associated with pork quality. 132 (Landrace × Yorkshire) × Duroc fatteners were genotyped by originally developed PCR-RFLP method. Upon the slaughter
Naohiro Kurotaki et al.
PloS one, 6(5), e20589-e20589 (2011-06-10)
The recent development of high-resolution DNA microarrays, in which hundreds of thousands of single nucleotide polymorphisms (SNPs) are genotyped, enables the rapid identification of susceptibility genes for complex diseases. Clusters of these SNPs may show runs of homozygosity (ROHs) that
Synthesis of ATP derivatives of compounds of the mevalonate pathway (isopentenyl di-and triphosphate; geranyl di-and triphosphate, farnesyl di-and triphosphate, and dimethylallyl diphosphate) catalyzed by T4 RNA ligase, T4 DNA ligase and other ligases: Potential relationship with the effect of bisphosphonates on osteoclasts
Sillero MAG, et al.
Biochemical Pharmacology, 78(4), 335-343 (2009)
Michael Veit et al.
Methods in molecular biology (Clifton, N.J.), 446, 163-182 (2008-04-01)
Palmitoylation or S-acylation is the post-translational attachment of fatty acids to cysteine residues and is common among integral and peripheral mem brane proteins. Palmitoylated proteins have been found in every eukaryotic cell type examined (yeast, insect, and vertebrate cells), as
Structural snapshots for the conformation-dependent catalysis by human medium-chain acyl-coenzyme A synthetase ACSM2A
Kochan G, et al.
Journal of Molecular Biology, 388(5), 997-1008 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service