MAB8702
Anti-Dengue Virus Type II Antibody, clone 3H5-1
clone 3H5-1, Chemicon®, from mouse
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Recommended Products
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
clone
3H5-1, monoclonal
species reactivity
human
manufacturer/tradename
Chemicon®
technique(s)
immunofluorescence: suitable
inhibition assay: suitable (hemagglutination)
isotype
IgG1
shipped in
wet ice
General description
Dengue fever is an acute, mosquito-transmitted viral disease characterized by fever, headache, arthralgia (severe retro-orbital pain), myalgia, rash, nausea, and vomiting. Infections are caused by any of the four closely related, but antigenically distinct virus serotypes (DEN-1, DEN-2, DEN-3, and DEN-4). Infection with one of these serotypes does not provide cross-protective immunity, so persons living in a dengue-endemic area can have four dengue infections during their lifetimes. Dengue is primarily an urban disease of the tropics, and the viruses that cause it are maintained in a cycle that involves humans and Aedes aegypti, a domestic, day-biting mosquito that prefers to feed on humans. Although most dengue infections result in relatively mild illness, some can produce Dengue Hemorrhagic Fever (DHF) or dengue shock syndrome, with children being particularly at risk. Although epidemic outbreaks have been reported since 1779, the incidence has been increasing, with global, multiple serotype pandemics intensifying within the last 15 years. There is no specific antiviral therapy for dengue, but for both classical dengue and dengue hemorrhagic fever, symptomatic and supportive measures are effective. Important risk factors for DHF include the strain and serotype of the virus involved, as well as the age, immune status, and genetic predisposition of the patient.
Specificity
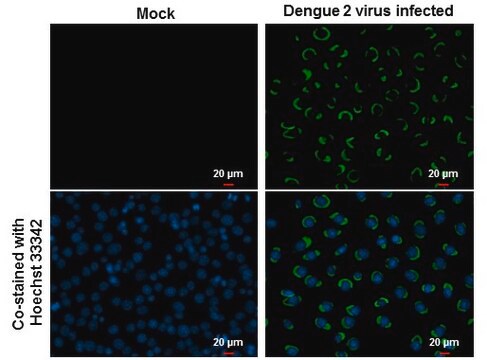
Reacts with the Dengue type 2 virus.
Immunogen
Dengue type 2 antigen (New Guinea C).
Application
Recommended for use in an immunofluorescent assay. Also suitable for use in hemagglutination-inhibition tests and plaque-reduction assays.
IFA: 1:200-1:400.
Dilute with buffer pH 7.5-8.0 to desired working volumes. For extensive dilution, protein containing or other stabilizing medium should be used.
Final working dilutions must be determined by end user.
IFA: 1:200-1:400.
Dilute with buffer pH 7.5-8.0 to desired working volumes. For extensive dilution, protein containing or other stabilizing medium should be used.
Final working dilutions must be determined by end user.
Research Category
Infectious Diseases
Infectious Diseases
Research Sub Category
Infectious Diseases - Viral
Infectious Diseases - Viral
This Anti-Dengue Virus Type II Antibody, clone 3H5-1 is validated for use in HI, IF for the detection of Dengue Virus Type II.
Physical form
Format: Purified
Protein G Purified
Protein G Purified immunoglobulin. Liquid in 0.02 M PB, 0.25 M NaCl, pH = 7.6 with 0.1% Na Azide as a preservative
Storage and Stability
Maintain at 2°C to 8°C in undiluted aliquots for up to 12 months after date of receipt.
Analysis Note
Control
Dengue positive patient sample
Dengue positive patient sample
Other Notes
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Zhi-Shan Zhang et al.
Molecular medicine reports, 11(2), 1009-1016 (2014-11-06)
There is currently no effective vaccine to prevent dengue infection, despite the existence of multiple studies on potential methods of immunization. The aim of the present study was to explore the effect of DNA and/or recombinant protein on levels of
Jorge I Castañeda-Sánchez et al.
Intervirology, 59(1), 8-19 (2016-06-20)
The innate immune response is remarkably important for controlling infections. Information about the participation of antimicrobial peptides (AMPs) in response to dengue virus (DENV) is scarce. The aim of this study was to examine the AMP response to DENV-2 in
Sialic acid expression in the mosquito Aedes aegypti and its possible role in dengue virus-vector interactions.
Cime-Castillo, J; Delannoy, P; Mendoza-Hernandez, G; Monroy-Martinez, V; Harduin-Lepers et al.
BioMed Research International null
Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking.
van der Schaar, HM; Rust, MJ; Waarts, BL; van der Ende-Metselaar, H; Kuhn, RJ; Wilschut et al.
Journal of virology null
Leticia Franco et al.
PLoS neglected tropical diseases, 5(8), e1251-e1251 (2011-08-11)
Dengue virus (DENV) circulates in human and sylvatic cycles. Sylvatic strains are both ecologically and evolutionarily distinct from endemic viruses. Although sylvatic dengue cycles occur in West African countries and Malaysia, only a few cases of mild human disease caused
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service