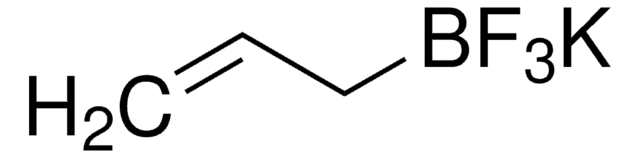
About This Item
Recommended Products
Assay
95%
form
solid
mp
>300 °C (lit.)
SMILES string
[K+].F[B-](F)(F)Cc1ccccc1
InChI
1S/C7H7BF3.K/c9-8(10,11)6-7-4-2-1-3-5-7;/h1-5H,6H2;/q-1;+1
InChI key
WGHDAVWURFVTQC-UHFFFAOYSA-N
Application
- Oxidation
- Ritter-type amidation with nitriles
- Photo-allylation / photo-benzylation of carbonyl compounds
- Stereoselective nucleophilic addition
- Suzuki cross-coupling
Organotrifluoroborates as versatile and stable boronic acid surrogates
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Bench-stable Potassium Organotrifluoroborates enable diverse C-C bond formation reactions.
Bench-stable Potassium Organotrifluoroborates enable diverse C-C bond formation reactions.
Bench-stable Potassium Organotrifluoroborates enable diverse C-C bond formation reactions.
Bench-stable Potassium Organotrifluoroborates enable diverse C-C bond formation reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service