All Photos(1)
About This Item
Linear Formula:
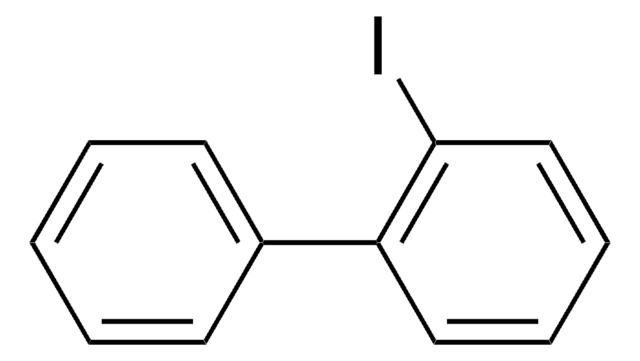
ClC6H3(I)CH3
CAS Number:
Molecular Weight:
252.48
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.624 (lit.)
bp
239 °C (lit.)
density
1.81 g/mL at 25 °C (lit.)
SMILES string
Cc1cc(I)ccc1Cl
InChI
1S/C7H6ClI/c1-5-4-6(9)2-3-7(5)8/h2-4H,1H3
InChI key
MMBDKGFWRIYSRD-UHFFFAOYSA-N
General description
2-Chloro-5-iodotoluene is a halogenated hydrocarbon.
Application
2-Chloro-5-iodotoluene may be used to synthesize:
- 1-(4-chloro-3-methylphenyl)-1H-pyrrolo[2,3-c]pyridine
- bromomethyl-2-chloro-5-iodobenzene
- 3-(4′-chloro-3′-tolyl)thiophene
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and characterization of all-conjugated graft copolymers comprised of n-type or p-type backbones and poly (3-hexylthiophene) side chains.
Wang J, et al.
Macromolecules, 46(5), 1783-1793 (2013)
Frédéric Stauffer et al.
Bioorganic & medicinal chemistry letters, 22(5), 1860-1863 (2012-02-18)
Aromatase inhibition is the new standard of care for estrogen receptor positive breast cancer and has also potential for treatment of other diseases such as endometriosis. Simple and readily available 3-pyridyl arylethers and 1-aryl pyrrolo[2,3-c]pyridines recapitulating the key pharmacophore elements
Susanna Tchilibon et al.
Journal of medicinal chemistry, 48(6), 1745-1758 (2005-03-18)
A series of ring-constrained (N)-methanocarba-5'-uronamide 2,N(6)-disubstituted adenine nucleosides have been synthesized via Mitsunobu condensation of the nucleobase precursor with a pseudosugar ring containing a 5'-ester functionality. Following appropriate functionalization of the adenine ring, the ester group was converted to the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service