All Photos(1)
About This Item
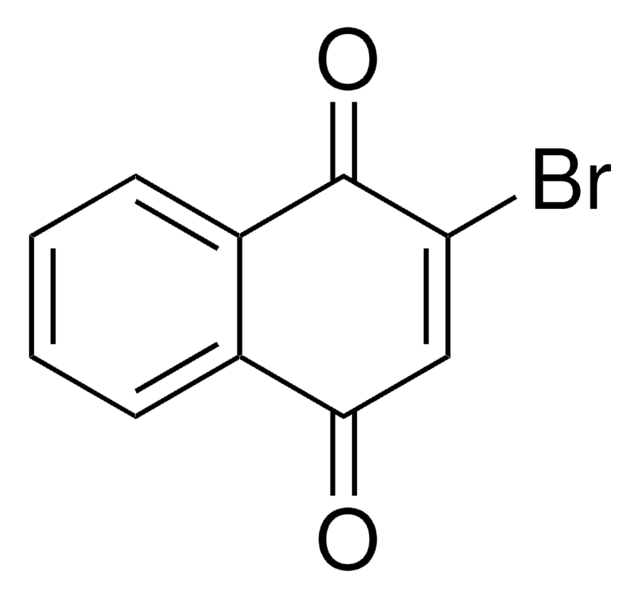
Empirical Formula (Hill Notation):
C10H4Br2O2
CAS Number:
Molecular Weight:
315.95
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
218-222 °C (lit.)
SMILES string
BrC1=C(Br)C(=O)c2ccccc2C1=O
InChI
1S/C10H4Br2O2/c11-7-8(12)10(14)6-4-2-1-3-5(6)9(7)13/h1-4H
InChI key
PSMABVOYZJWFBV-UHFFFAOYSA-N
Related Categories
General description
2,3-Dibromo-1,4-naphthoquinone is a 2,3-disubstituted 1,4-naphthoquinone. It is a plumbagin derivative and an acaricide. It undergoes photochemical reaction with 2-methoxy-1-alkene to yield derivatives of 2-(2-alkanonyl)-1,4-naphthoquinone.
Application
2,3-Dibromo-1,4-naphthoquinone may be used in the synthesis of 3-[3-(2-carboxy-ethylsulfanyl)-1,4-dioxo-1,4-dihydro-naphthalen-2-ylsulfanyl]-propionic acid and NSC 95397 (a protein tyrosine phosphatase antagonist).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Vincent P Peyregne et al.
Molecular cancer therapeutics, 4(4), 595-602 (2005-04-14)
Cdc25 phosphatases are important in cell cycle control and activate cyclin-dependent kinases (Cdk). Efforts are currently under way to synthesize specific small-molecule Cdc25 inhibitors that might have anticancer properties. NSC 95397, a protein tyrosine phosphatase antagonist from the National Cancer
A one-pot synthesis of 1, 4-naphthoquinone-2, 3-bis-sulfides catalysed by a commercial laccase.
Wellington KW, et al.
Green Chemistry, 14(9), 2567-2576 (2012)
Chi-Hoon Lee et al.
Journal of microbiology and biotechnology, 18(2), 314-321 (2008-03-01)
Acaricidal effects of materials derived from Diospyros kaki roots against Dermatophagoides farinae and D. pteronyssinus were assessed using impregnated fabric disk bioassay and compared with that of the commercial benzyl benzoate. The observed responses varied according to dosage and mite
A new method of ?-keto alkyl chain introduction into 1, 4-naphthoquinone.
Maruyama K, et al.
Chemistry Letters (Jpn), 13(3), 371-374 (1984)
Xiao-Fei Shang et al.
Scientific reports, 8(1), 1609-1609 (2018-01-27)
As important secondary plant metabolites, naphthoquinones exhibit a wide range of biological activities. However, their potential as sustainable alternatives to synthetic acaricides has not been studied. This study for the first time investigates the acaricidal activity of naphthoquinones against Psoroptes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service