456772
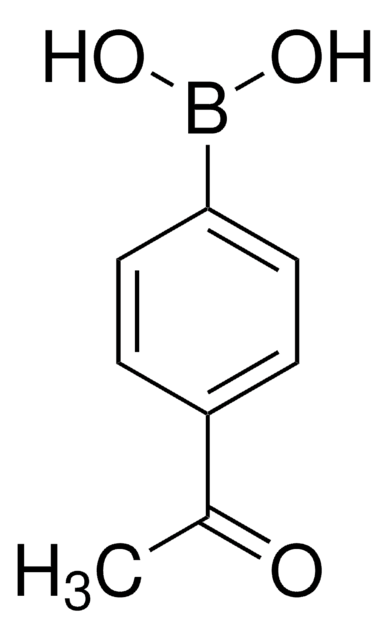
4-Carboxyphenylboronic acid
Synonym(s):
4-(Dihydroxyboronyl)benzoic acid, 4-(Dihydroxyboryl)benzoic acid, 4-Boronobenzoic acid, 4-Carboxybenzeneboronic acid, 4-Carboxylphenylboronic acid, 4-Hydroxycarbonylphenyl boronic acid, NSC 221170, p-Boronobenzoic acid, p-Carboxybenzeneboronic acid, p-Carboxyphenylboronic acid
About This Item
Recommended Products
mp
220 °C (dec.) (lit.)
SMILES string
OB(O)c1ccc(cc1)C(O)=O
InChI
1S/C7H7BO4/c9-7(10)5-1-3-6(4-2-5)8(11)12/h1-4,11-12H,(H,9,10)
InChI key
SIAVMDKGVRXFAX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Condensation reactions with stabilizer chains at the surface of polystyrene latex
- Suzuki coupling reactions
- Esterification
- Derivatization of polyvinylamine
- Synthesis of isotopically labeled mercury
- Functionalization of poly-SiNW for detection of dopamine
- Suzuki-Miyaura cross-coupling
- Induction of pH sensitivity on fluorescence lifetime of quantum dots by NIR fluorescent dyes
- Bio-supported palladium nanoparticles as phosphine-free catalyst for Suzuki reaction in water
- Chan-Lam-type Copper (Cu)-catalyzed S-arylation with aryl boronic acids at room temperature
Reagent used in Preparation of
- Isoquinolones via regioselective Suzuki-Miyaura cross-coupling and tandem palladium-catalyzed intramolecular aminocarbonylation and annulation
- Amprenavir-based P1-substituted bi-aryl derivatives as ultra-potent HIV-1 protease inhibitors
- Phenols via visible-light initiated aerobic oxidative hydroxylation of arylboronic acids using air as oxidant catalyzed by Ruthenium (Ru)-complex
- Glucose sensitive boronic acid-bearing block copolymers
- Trisulfonated calixarene upper-rim sulfonamido derivatives and their complexation with the trimethyllysine epigenetic mark
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service