All Photos(1)
About This Item
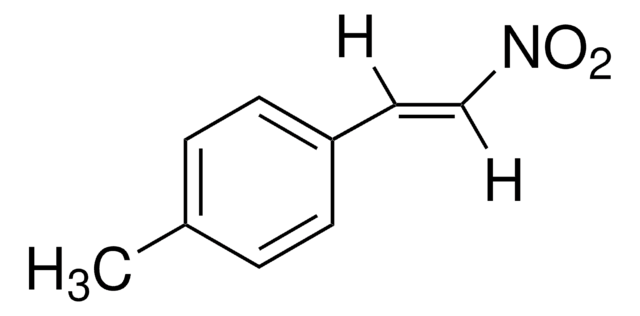
Linear Formula:
CH3OC6H4CH=CHNO2
CAS Number:
Molecular Weight:
179.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
86-88 °C (lit.)
solubility
chloroform: soluble 25 mg/mL, clear, yellow
SMILES string
[H]\C(=C(\[H])[N+]([O-])=O)c1ccc(OC)cc1
InChI
1S/C9H9NO3/c1-13-9-4-2-8(3-5-9)6-7-10(11)12/h2-7H,1H3/b7-6+
InChI key
JKQUXSHVQGBODD-VOTSOKGWSA-N
Related Categories
General description
trans-4-Methoxy-β-nitrostyrene participates in the Michael reaction on carbapenam intermediate.
Application
trans-4-Methoxy-β-nitrostyrene acts as guest molecule and forms guest molecules forming co-crystal phases with the d-form of robust syndiotactic polystyrene (sPS). It may be employed as a Michael acceptor in the synthesis of proline based chiral ionic liquid catalysts with two five-membered unsaturated aza-heterocycles.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Polymer co-crystalline films for photonics.
Daniel C, et al.
Journal of the European Optical Society: Rapid Publications, 4 (2009)
Proline Based Chiral Ionic Liquids for Enantioselective Michael Reaction.
Nobuoka K, et al.
Organic Chemistry International (2014)
Sachin A Pawar et al.
Organic & biomolecular chemistry, 11(48), 8294-8297 (2013-11-13)
Herein, we report the development of mild, organocatalyzed routes to novel carbapenam derivatives through aldol, Mannich and Michael C-C bond forming reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service