All Photos(3)
About This Item
Empirical Formula (Hill Notation):
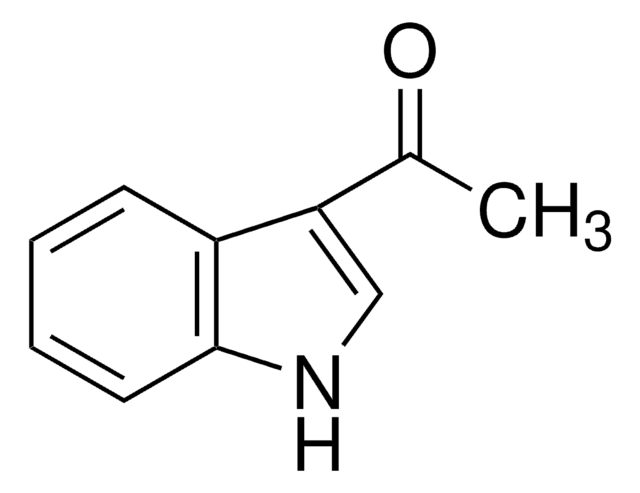
C10H9NO
CAS Number:
Molecular Weight:
159.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.607 (lit.)
bp
123-125 °C/8 mmHg (lit.)
density
1.387 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(=O)n1ccc2ccccc12
InChI
1S/C10H9NO/c1-8(12)11-7-6-9-4-2-3-5-10(9)11/h2-7H,1H3
InChI key
UUCUQJHYUPXDHN-UHFFFAOYSA-N
General description
Quantum chemical calculations of ground state energy, geometrical structure and vibrational wavenumbers of 1-acetylindole has been carried out using density functional (DFT/B3LYP) method. Regioselective acylations of 1-acetylindole (N-acetylindole) under Friedel-Crafts reaction has been reported. Reaction of 1-acetylindole with manganese(III) acetate in the presence of malonic acid, is reported to afford 4-acetyl-3,3a,4,8b-tetrahydro-2H-furo[3,2-b]indol-2-one.
Application
1-Acetylindole may be used in the stereocontrolled synthesis of (±)-geissoschizine. It may be used in the preparation of (1-acetyl-κO-indolyl-κC2)tetracarbonylmanganese, via a standard cyclomanganation procedure.
Reactant for preparation of:
Reactant for:
- Antimycobacterial agents
- Cyclin-dependent kinase (CDK2) inhibitors
Reactant for:
- C3-C3 oxidative cross-coupling reactions
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and alkyne-coupling chemistry of cyclomanganated 1-and 3-acetylindoles, 3-formylindole and analogues.
Depree GJ, et al.
Journal of Organometallic Chemistry, 691(4), 667-679 (2006)
Vikas K Shukla et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 133, 626-638 (2014-07-06)
Quantum chemical calculations of ground state energy, geometrical structure and vibrational wavenumbers of 1-acetylindole were carried out using density functional (DFT/B3LYP) method with 6-311++G(d,p) basis set. The FT-IR and FT-Raman spectra were recorded in the condensed state. The fundamental vibrational
Mangenese (III) acetate oxidation of 1-acetylindole derivatives.
Izumi T, et al.
Journal of Heterocyclic Chemistry, 30(4), 1133-1136 (1993)
A concise, stereoselective synthesis of (?)-geissoschizine.
Bennasar M, et al.
Tetrahedron Letters, 37(50), 9105-9106 (1996)
Regioselective acylations at the 2 and 6 position of N-acetylindole.
Cruz R, et al.
Tetrahedron Letters, 42(8), 1467-1469 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service