340200
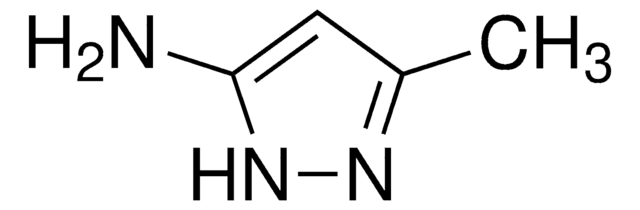
3-Amino-5-methylpyrazole
97%
Synonym(s):
5-Methyl-3-pyrazolamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H7N3
CAS Number:
Molecular Weight:
97.12
Beilstein:
1904
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
bp
213 °C/14 mmHg (lit.)
mp
45-47 °C (lit.)
SMILES string
Cc1cc(N)n[nH]1
InChI
1S/C4H7N3/c1-3-2-4(5)7-6-3/h2H,1H3,(H3,5,6,7)
InChI key
FYTLHYRDGXRYEY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Complexation of the amino- and carboxyl-protected tripeptide with 3-amino-5-methylpyrazole was studied by low-temperature NMR experiments in a freonic solvent.
Application
3-Amino-5-methylpyrazole was employed as beta-sheet template to investigate its interaction with ferrocenoyl-dipeptides. It was also used in the synthesis of 4-aryl-3,7,7-trimethyl-2,4,6,7,8,9-hexahydropyrazolo[3,4-b]quinolin-5-ones.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Wei Wang et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 13(3), 854-861 (2006-11-07)
Complexation of the amino- and carboxyl-protected tripeptide Piv-L-Val-L-Val-L-Val-tBu with 3-methylpyrazole and 3-amino-5-methylpyrazole was studied by low-temperature NMR experiments in a freonic solvent. The peptide forms an extended beta-type structure at all temperatures and associates through hydrogen bonding with the two
P Saweczko et al.
Inorganic chemistry, 40(17), 4409-4419 (2001-08-07)
The use of 3-aminopyrazole derivatives as beta-sheet templates is investigated using a series of ferrocenoyl (Fc)-dipeptides (Fc-Gly(2)-OEt, Fc-Ala(2)-OBzl, Fc-Leu-Phe-OMe, Fc-Val-Phe-OMe, Fc-Phe(2)-OMe, Fc-Leu(2)-OMe, Fc-Val(2)-OMe). The synthesis and full characterization are reported. The solid-state structures of Fc-Gly(2)-OMe and Fc-Leu-Phe-OMe show extensive hydrogen
Synthesis of partially hydrogenated pyrazolo [3, 4-b] quinolinones by condensation of 3-amino-5-methylpyrazole with aromatic aldehydes and dimedone.
Lipson VV, et al.
Russ. J. Org. Chem., 42(7), 1015-1021 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service