All Photos(1)
About This Item
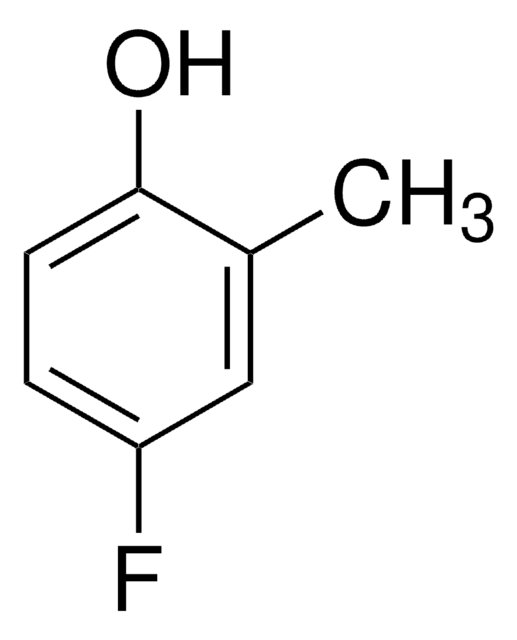
Linear Formula:
FC6H3(CH3)OH
CAS Number:
Molecular Weight:
126.13
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.515 (lit.)
bp
76 °C/5 mmHg (lit.)
mp
32 °C (lit.)
density
1.134 g/mL at 25 °C (lit.)
SMILES string
Cc1cc(O)ccc1F
InChI
1S/C7H7FO/c1-5-4-6(9)2-3-7(5)8/h2-4,9H,1H3
InChI key
RVYGYYVGWSCWGY-UHFFFAOYSA-N
Application
4-Fluoro-3-methylphenol was used to detect the aromatic metabolites in methanogenic m-cresol-degrading consortium. It was also used in the preparation of 8-fluoronaphthoquinone via Friedel Crafts acylation reaction with maleic anhydride.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
206.6 °F - closed cup
Flash Point(C)
97 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
K L Londry et al.
Applied and environmental microbiology, 59(7), 2229-2238 (1993-07-01)
Anaerobic sewage sludge was used to enrich a methanogenic m-cresol-degrading consortium. 6-Fluoro-3-methylphenol was synthesized and added to subcultures of the consortium with m-cresol. This caused the accumulation of 4-hydroxy-2-methylbenzoic acid. In a separate experiment, the addition of 3-fluorobenzoic acid caused
Anita Mahapatra et al.
Bioorganic & medicinal chemistry, 15(24), 7638-7646 (2007-09-25)
The naphthoquinone 7-methyljuglone (5-hydroxy-7-methyl-1,4-naphthoquinone) has previously been isolated and identified as an active component of root extracts of Euclea natalensis which displays antitubercular activity. Herein, a series of synthetic and plant-derived naphthoquinone derivates of the 7-methyljuglone scaffold have been prepared
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service