All Photos(1)
About This Item
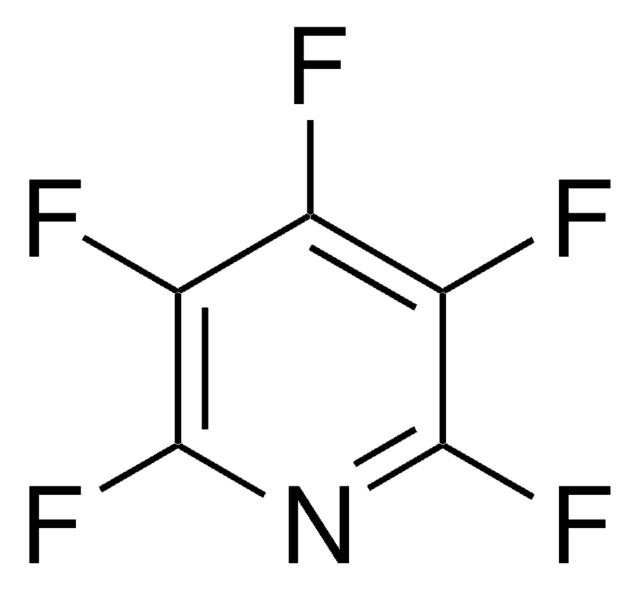
Linear Formula:
C6F5NO2
CAS Number:
Molecular Weight:
213.06
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.447 (lit.)
bp
158-161 °C (lit.)
density
1.656 g/mL at 25 °C (lit.)
SMILES string
[O-][N+](=O)c1c(F)c(F)c(F)c(F)c1F
InChI
1S/C6F5NO2/c7-1-2(8)4(10)6(12(13)14)5(11)3(1)9
InChI key
INUOFQAJCYUOJR-UHFFFAOYSA-N
General description
Electron attachment to pentafluoronitrobenzene has been studied in the energy range 0-16eV by means of a crossed electron-molecular beam experiment with mass spectrometric detection of the anions. The electroreduction of pentafluoronitrobenzene in dimethylformamide solution results in the formation of the dimer, octafluoro-4,4′-dinitro-biphenyl.
Application
Pentafluoronitrobenzene has been used in the preparation of p-azidotetrafluoroaniline, a new photoaffinity reagent.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
195.8 °F - closed cup
Flash Point(C)
91 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
K A Chehade et al.
The Journal of organic chemistry, 65(16), 4949-4953 (2000-08-24)
p-Azidotetrafluoroaniline (1) was synthesized in 65-73% yield by two different methods employing a stable carbamate intermediate. The first method trapped the intermediate isocyanate generated via a modified Curtius rearrangement with 2-methyl-2-propanol or 2-(trimethylsilyl)ethanol to form the stable carbamates 2d and
Voltammetry under high mass transport conditions. The application of the high speed channel electrode to the reduction of pentafluoronitrobenzene.
Coles BA, et al.
Journal of Electroanalytical Chemistry, 411(1), 121-127 (1996)
Judith Langer et al.
Physical chemistry chemical physics : PCCP, 10(11), 1523-1531 (2008-03-11)
Electron attachment to pentafluorobenzonitrile (C(6)F(5)CN) and pentafluoronitrobenzene (C(6)F(5)NO(2)) is studied in the energy range 0-16 eV by means of a crossed electron-molecular beam experiment with mass spectrometric detection of the anions. We find that pentafluoronitrobenzene exclusively generates fragment anions via
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service