MABF954
Anti-LAG3 Antibody, clone 4-10-C9
clone 4-10-C9, from mouse
Synonym(s):
Lymphocyte activation gene 3 protein, CD223, LAG-3
About This Item
Recommended Products
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
4-10-C9, monoclonal
species reactivity
mouse
technique(s)
flow cytometry: suitable
immunocytochemistry: suitable
isotype
IgG2aκ
NCBI accession no.
UniProt accession no.
shipped in
wet ice
target post-translational modification
unmodified
Gene Information
mouse ... Lag3(16768)
General description
Specificity
Immunogen
Application
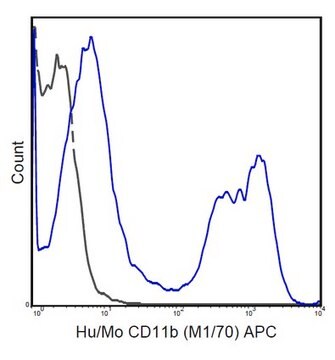
Flow Cytometry Analysis: A representative lot was fluorescently conjugated and detected an increased number of LAG-3-positive cells within the CD4+ and CD8+ populations of infiltrating lymphocytes (TILs) in tumors developed in mice exografted with murine B16 melanoma, MC38 colon adenocarcinoma, or Sa1N fibrosarcoma cells (Woo, S.R., et al. (2012). Cancer Res. 72(4): 917–927).
Flow Cytometry Analysis: A representative lot, pre-conjugated with Alexa Fluor™ 647, detected both surface and intracellular LAG-3 by immunofluorescent staining of non-permeabilized and permeabilized primary murine CD4+ T cells activated in vitro via CD3 & CD28 cross-linking by immobilized antibodies. Pronase treatment of cells prior to permeabilization abolished cell surface staining (Woo, S.R., et al. (2010). Eur. J. Immunol. 40(6):1768-1777).
Flow Cytometry Analysis: A representative lot, pre-conjugated with Alexa Fluor 647, detected a time-dependent recovery of cell surface LAG-3 immunoreactivity on activated murine CD4+ T cells after initial surface LAG-3 degradation by pronase treatment. Protein synthesis inhibitor cycloheximide (Cat. No. 239764) or protein transport inhibitor Brefeldin A (Cat. No. 203729) treatment partially blocked the recovery (Woo, S.R., et al. (2010). Eur. J. Immunol. 40(6):1768-1777).
Immunocytochemistry Analysis: A representative lot detected both surface and intracellular LAG-3 by fluorescent immunocytochemistry staining of non-permeabilized and permeabilized primary murine CD4+ T cells activated in vitro via CD3 & CD28 cross-linking by immobilized antibodies. Pronase treatment of cells prior to permeabilization abolished cell surface staining (Woo, S.R., et al. (2010). Eur. J. Immunol. 40(6):1768-1777).
Immunocytochemistry Analysis: A representative lot detected intracellular LAG-3 immunoreactivity co-localized with those of the early and recycling endosome marker EEA1, as well as endosomal markers Rab11b and Rab27a by fluorescent immunocytochemistry staining of activated murine CD4+ T cells following pronase treatment and permeabilization (Woo, S.R., et al. (2010). Eur. J. Immunol. 40(6):1768-1777).
Quality
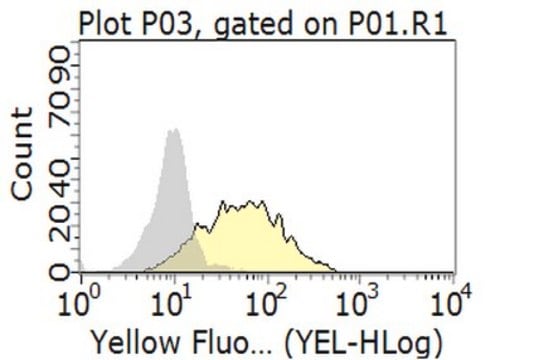
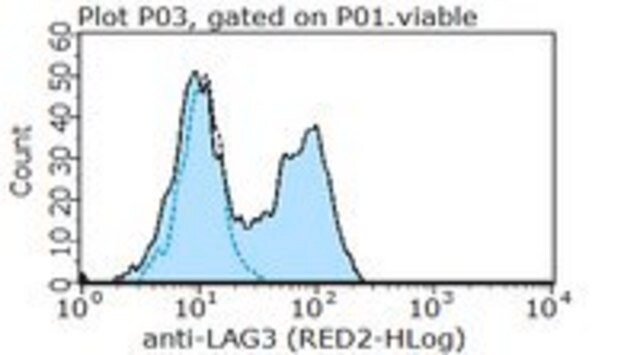
Flow Cytometry Analysis: 1 µg/mL of this antibody detected an induction of LAG-3-positive population in isolated mouse splenocytes following a 3-day 2 µg/mL Concanavalin A (Con A) stimulation.
Target description
Physical form
Legal Information
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service