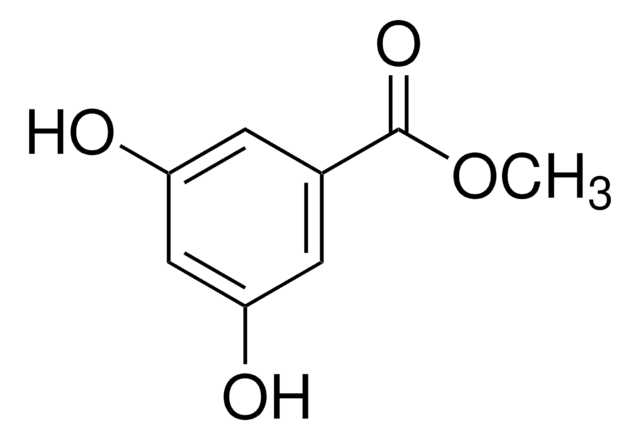
D110000
3,5-Dihydroxybenzoic acid
97%
Synonym(s):
α-Resorcylic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(HO)2C6H3CO2H
CAS Number:
Molecular Weight:
154.12
Beilstein:
2207864
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
236-238 °C (dec.) (lit.)
SMILES string
OC(=O)c1cc(O)cc(O)c1
InChI
1S/C7H6O4/c8-5-1-4(7(10)11)2-6(9)3-5/h1-3,8-9H,(H,10,11)
InChI key
UYEMGAFJOZZIFP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
392.0 °F - closed cup
Flash Point(C)
200 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Silke C Wenzel et al.
Chembiochem : a European journal of chemical biology, 9(16), 2711-2721 (2008-10-31)
Kendomycin is a bioactive polyketide that is produced by various Streptomyces strains. It displays strong antibiotic activities against a wide range of bacteria and exhibits remarkable cytotoxic effects on the growth of several human cancer cell lines. In this study
Seetharaman Vaidyanathan et al.
Rapid communications in mass spectrometry : RCM, 20(8), 1192-1198 (2006-03-17)
Matrix-assisted laser desorption/ionisation (MALDI) mass spectrometry was investigated for the simultaneous detection of several metabolites, as applicable to global metabolite analysis (metabolomics). The commonly employed organic matrices alpha-cyano-4-hydroxycinnamic acid and 3,5-dihydroxybenzoic acid, in both the crystalline and ionic liquid forms
Abigail E Wolfe et al.
Biochemistry, 46(19), 5741-5753 (2007-04-21)
Dihydroorotate dehydrogenases (DHODs) catalyze the oxidation of dihydroorotate to orotate in the only redox reaction in pyrimidine biosynthesis. The pyrimidine binding sites are very similar in all structurally characterized DHODs, suggesting that the prospects for identifying a class-specific inhibitor directed
Yasuyo Seshime et al.
Bioorganic & medicinal chemistry letters, 20(16), 4785-4788 (2010-07-16)
As a novel superfamily of type III polyketide synthases in microbes, four genes csyA, csyB, csyC, and csyD, were found in the genome of Aspergillus oryzae, an industrially important filamentous fungus. In order to analyze their functions, we carried out
Anja Koskela et al.
Clinical chemistry, 53(7), 1380-1383 (2007-05-15)
Whole-grain rye and wheat cereals contain high amounts of alkylresorcinols (ARs), phenolic lipids. ARs can be quantified in plasma. Two recently identified urinary AR metabolites, 3,5-dihydroxyphenylbenzoic acid (DHBA) and 3-(3,5-dihydroxyphenyl)-1-propanoic acid (DHPPA), may be useful as biomarkers of intake of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service