559091
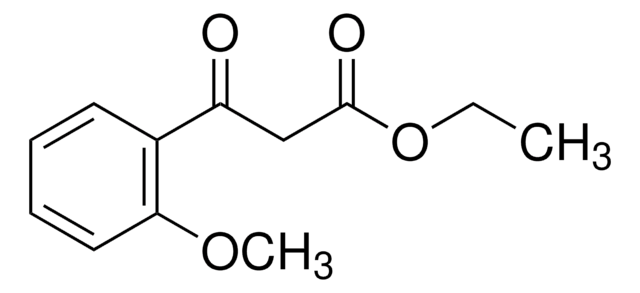
Ethyl (2-chlorobenzoyl)acetate
≥95%
Synonym(s):
Ethyl (o-chlorobenzoyl)acetate, Ethyl 3-(2-chlorophenyl)-3-oxopropanoate, NSC 158136
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClC6H4COCH2CO2C2H5
CAS Number:
Molecular Weight:
226.66
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
refractive index
n20/D 1.540 (lit.)
bp
221-222 °C (lit.)
density
1.206 g/mL at 25 °C (lit.)
functional group
chloro
ester
ketone
SMILES string
CCOC(=O)CC(=O)c1ccccc1Cl
InChI
1S/C11H11ClO3/c1-2-15-11(14)7-10(13)8-5-3-4-6-9(8)12/h3-6H,2,7H2,1H3
InChI key
DLFBNTUSDQSFOF-UHFFFAOYSA-N
Application
Ethyl (2-chlorobenzoyl)acetate may be used to synthesize ketosplitomicin derivative and ethyl 6-(2-chlorophenyl)-2,2-dimethyl-4-oxo-2,3-dihydro-4Hpyran-5-carboxylate.
Reactant for:
- Cerium ammonium nitrate-mediated oxidative coupling
- Hydrosilylation reactions
- Preparation of diaryl-substituted pyrazoles as potent CCR2 receptor antagonists
- Preparation of potential herbicidal agents
- Ruthenium-catalyzed asymmetric hydrogenation
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Simple access to 5-carboalkoxy-2, 3-dihydro-4H-pyran-4-ones via domino acylative electrocyclization: the first three step total synthesis of the dihydronaphthopyran-4-one class of natural products.
Ilangovan A and Sakthivel P.
Royal Society of Chemistry Advances, 4(98), 55150-55161 (2014)
Characterization of sirtuin inhibitors in nematodes expressing a muscular dystrophy protein reveals muscle cell and behavioral protection by specific sirtinol analogues.
Pasco MY, et al.
Journal of Medicinal Chemistry, 53(3), 1407-1411 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service