471208
Ethylamine solution
66.0-72.0% in H2O
Synonym(s):
Aminoethane, Monoethylamine
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
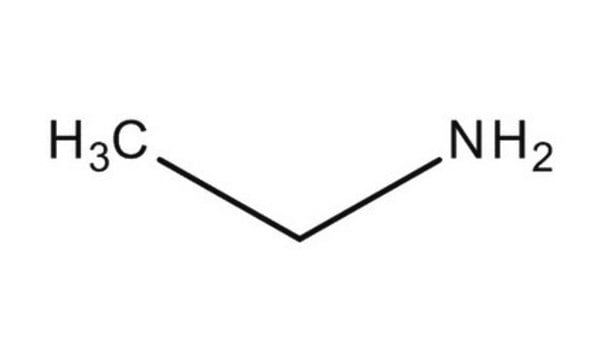
C2H5NH2
CAS Number:
Molecular Weight:
45.08
Beilstein:
505933
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
concentration
66.0-72.0% in H2O
density
0.81 g/mL at 20 °C
functional group
amine
SMILES string
CCN
InChI
1S/C2H7N/c1-2-3/h2-3H2,1H3
InChI key
QUSNBJAOOMFDIB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Ethylamine solution is used in the preparation of N-ethyl triazineepiperazine copolymer (ETPC), as a hydrophobic flame retardant material. It is also used in the modification of poly(tetramethylene oxide)/silica hybrid composites.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
71.6 °F
Flash Point(C)
22 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of N-ethyl triazine-piperazine copolymer and flame retardancy and water resistance of intumescent flame retardant polypropylene.
Yang, Kun et al.
Polymer Degradation and Stability, 98(7), 1397-1406 (2013)
Modification of an inorganic-organic hybrid composite: effect of ethylamine.
Brennan, Anthony B and Miller, Thomas M
Chemistry of Materials, 6(3), 262-267 (1994)
Masayasu Taki et al.
Biochemistry, 47(29), 7726-7733 (2008-07-17)
During the catalytic reaction of copper amine oxidase, one of the two prochiral hydrogen atoms at the C1 position of substrate amine is stereoselectively abstracted by a conserved Asp residue serving as a general base. Using stereospecifically deuterium-labeled enantiomers of
Aurore Thibon et al.
Dalton transactions (Cambridge, England : 2003), (43)(43), 9587-9594 (2009-10-28)
The new ligand L(6)(2)4E (N,N,N',N'-tetrakis(5-ethyl-2-pyridylmethyl)ethane-1,2-diamine) was designed as a more robust analog of TPEN (N,N,N',N'-tetrakis(2-pyridylmethyl)ethane-1,2-diamine) for which the ability at stabilizing high valent Fe-Oxo and Fe-(hydro)peroxo has been reported. With respect to the latter, the pyridyl beta-substituents in L(6)(2)4E do
Câline Christine et al.
Organic & biomolecular chemistry, 10(46), 9183-9190 (2012-10-23)
The synthesis of a phosphonated acyclic bifunctional chelate L* for the labeling of biomaterial is described. L* is based on a pyridine backbone, functionalized in ortho positions by aminomethyl-bis-methylphosphonic acids, and, in the para position, by a side chain containing
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service