All Photos(2)
About This Item
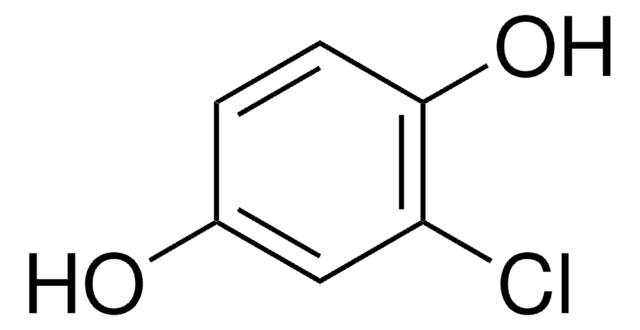
Linear Formula:
BrC6H3(OH)2
CAS Number:
Molecular Weight:
189.01
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
112-116 °C (lit.)
SMILES string
Oc1ccc(O)c(Br)c1
InChI
1S/C6H5BrO2/c7-5-3-4(8)1-2-6(5)9/h1-3,8-9H
InChI key
REFDOIWRJDGBHY-UHFFFAOYSA-N
Application
Bromohydroquinone was used in the synthesis of Π-conjugated polymers composed of alkyl carbazole/dialkoxyphenylene and squaraine units via Sonogashira cross-coupling reactions. It was used in the preparation of 2-bromobenzoquinone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T J Monks et al.
Toxicology and applied pharmacology, 103(3), 557-563 (1990-05-01)
Glutathione (GSH) conjugates of 2-bromohydroquinone are more difficult to oxidize than the parent hydroquinone. Hydrolysis catalyzed by gamma-glutamyl transpeptidase (gamma-GT), however, results in the formation of the corresponding cysteine conjugate which is more readily oxidized than the parent hydroquinone. N-Acetylation
S S Lau et al.
Toxicology and applied pharmacology, 103(1), 121-132 (1990-03-15)
We have previously shown that the renal necrosis observed after 2-bromohydroquinone (2-BrHQ) administration to rats is probably caused by the formation of 2-Br-(diglutathion-S-yl)HQ (2-Br-[diGSyl]HQ), since injection of this conjugate caused severe proximal tubular necrosis. In the present study we report
S S Lau et al.
Drug metabolism and disposition: the biological fate of chemicals, 15(6), 801-807 (1987-11-01)
Homogenates from rat renal papillae, a rich source of the prostaglandin (PG) H synthase system (PHS), metabolized [14C]2-bromohydroquinone, in the presence of arachidonic acid, to products which are covalently bound to protein. The co-oxidation of 2-bromohydroquinone caused a concentration-dependent stimulation
D P Rodeheaver et al.
The Journal of pharmacology and experimental therapeutics, 256(3), 917-921 (1991-03-01)
The basis of extracellular acidosis amelioration of 2-bromohydroquinone (BHQ)-induced renal proximal tubular cell death was determined by comparing the metabolism, uptake and mitochondrial effects of BHQ (0.2 mM) and bromoquinone (BQ) (0.05 mM) on isolated rabbit renal proximal tubules incubated
Anuradha Ramoji et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 69(3), 926-932 (2007-07-07)
Vibrational spectral measurements, namely, infrared (4000-400 cm(-1)) and Raman (3500-50 cm(-1)) spectra have been made for 2-Bromohydroquinone. Optimized geometrical structures, harmonic vibrational frequencies and intensities have been computed by the ab initio (RHF), B-based (BLYP, BP86) and B3-based (B3P86, B3LYP
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service