135046
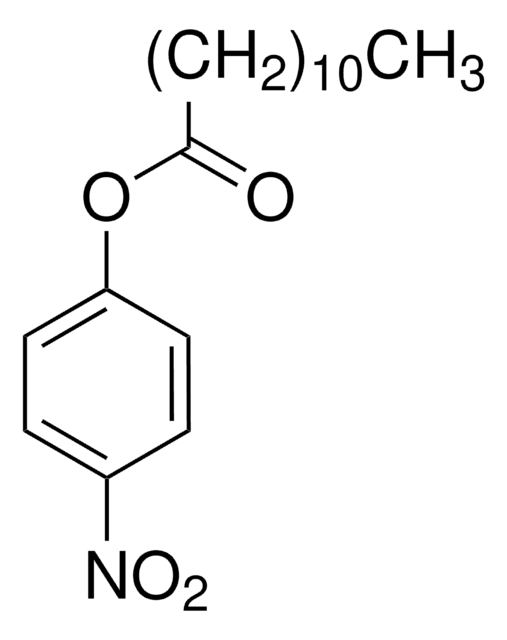
4-Nitrophenyl trimethylacetate
98%
Synonym(s):
4-Nitrophenyl pivalate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3CCO2C6H4NO2
CAS Number:
Molecular Weight:
223.23
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
92-95 °C (lit.)
SMILES string
CC(C)(C)C(=O)Oc1ccc(cc1)[N+]([O-])=O
InChI
1S/C11H13NO4/c1-11(2,3)10(13)16-9-6-4-8(5-7-9)12(14)15/h4-7H,1-3H3
InChI key
QADVJDGFQGNSIF-UHFFFAOYSA-N
General description
4-Nitrophenyl trimethylacetate undergoes hydrolysis catalysed by cytoplasmic aldehyde dehydrogenase in presence of NAD+, NADH, propionaldehyde, chloral hydrate, diethylstilboestrol and p-nitrobenzaldehyde (modifiers).
Application
4-Nitrophenyl trimethylacetate was used as sensitive probe to study the catalytic activity of carboxypeptidase Y.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The trimethylacetyl-transglutaminase complex.
J E Folk
Methods in enzymology, 87, 36-42 (1982-01-01)
T M Kitson
The Biochemical journal, 257(2), 573-578 (1989-01-15)
The effects of modifiers (NAD+, NADH, propionaldehyde, chloral hydrate, diethylstilboestrol and p-nitrobenzaldehyde) on the hydrolysis of p-nitrophenyl (PNP) pivalate (PNP trimethylacetate) catalysed by cytoplasmic aldehyde dehydrogenase are reported. In each case a different inhibition pattern is obtained to that observed
Studies of the esterase activity of cytosolic aldehyde dehydrogenase using sterically hindered and cyclic substrates.
K E Kitson et al.
Advances in experimental medicine and biology, 372, 45-52 (1995-01-01)
H C Margolis et al.
The Journal of biological chemistry, 253(21), 7891-7897 (1978-11-10)
In the course of our further investigation of the active site titration of carboxypeptidase Y, using 4-nitrophenyl trimethylacetate, we have found that carboxypeptidase Y can be isolated in different molecular forms. Carboxypeptidase Y obtained from Fleischmann baker's yeast has a
Roall Espersen et al.
Applied microbiology and biotechnology, 104(6), 2513-2522 (2020-01-29)
Two proteases, named C- and T-like proteases, respectively, were purified from the culture supernatant of Amycolatopsis keratinophila subsp. keratinophila D2T grown on a keratinous slaughterhouse by-product of pig bristles and nails as sole nitrogen and carbon source. The two proteases
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service