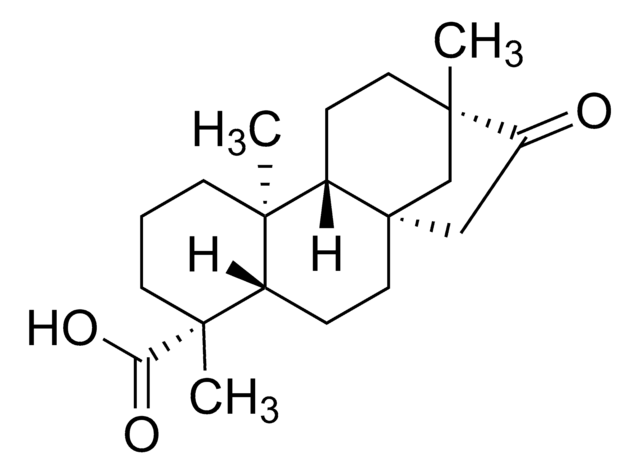
H8664
Steviol hydrate
≥98% (HPLC), powder
Synonym(s):
(4α)-13-Hydroxykaur-16-en-18-oic acid hydrate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H30O3 · xH2O
CAS Number:
Molecular Weight:
318.45 (anhydrous basis)
MDL number:
UNSPSC Code:
12161501
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Assay
≥98% (HPLC)
form
powder
color
white to tan
solubility
DMSO: >10 mg/mL
storage temp.
2-8°C
SMILES string
O.C[C@@]12CCC[C@](C)([C@H]1CC[C@]34CC(=C)[C@](O)(CC[C@@H]23)C4)C(O)=O
InChI
1S/C20H30O3.H2O/c1-13-11-19-9-5-14-17(2,7-4-8-18(14,3)16(21)22)15(19)6-10-20(13,23)12-19;/h14-15,23H,1,4-12H2,2-3H3,(H,21,22);1H2/t14-,15-,17+,18+,19+,20-;/m0./s1
InChI key
SGPAFVVDOPSBEU-CRRYNOPCSA-N
Biochem/physiol Actions
Steviol is an inhibitor of hOAT1 and hOAT3 organic anion transporters.
Steviol is an inhibitor of hOAT1 and hOAT3 organic anion transporters. Human organic anion transporter hOAT1 belongs to a superfamily of organic anion transporters, which play critical roles in the body disposition of clinically important drugs including anti-HIV therapeutics, anti-tumor drugs, antibiotics, anti-hypertensives, and anti-inflammatories. Steviol is a useful tool for studying renal drug clearance.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Motohiko Ukiya et al.
Chemistry & biodiversity, 10(2), 177-188 (2013-02-19)
Seventeen steviol derivatives, i.e., 2-18, and 19 isosteviol derivatives, i.e., 19-37, were prepared from a diterpenoid glycoside, stevioside (1). Upon evaluation of the cytotoxic activities of these compounds against leukemia (HL60), lung (A549), stomach (AZ521), and breast (SK-BR-3) cancer cell
Amal A A Mohamed et al.
Journal of plant physiology, 168(10), 1136-1141 (2011-04-12)
A short-term experiment was designed to measure the transcript levels of downstream genes contributing to the biosynthesis of steviol glycosides. Stevia rebaudiana plants were subjected to long- and short-day conditions for different time intervals. Samples from both lower and upper
J Isabella Zhang et al.
The Analyst, 137(13), 3091-3098 (2012-05-19)
Leaf spray mass spectrometry is explored as a fast and simple way for direct analysis of sweet glycosides in fresh untreated Stevia leaves without sample pretreatment. In this technique, a fresh triangular piece of Stevia leaf serves as both sample
M S Melis et al.
Brazilian journal of biology = Revista brasleira de biologia, 69(2), 371-374 (2009-08-14)
Stevia rebaudiana, a South American plant normally used as a natural herbal sweetener, has been suggested as exerting beneficial effects on human health, including as an antihypertensive and antihyperglycemic. The present experiment was undertaken to evaluate the renal excretion of
Hitesh Kumar et al.
Gene, 492(1), 276-284 (2011-11-01)
Stevia [Stevia rebuaidana (Bertoni); family: Asteraceae] is known to yield diterpenoid steviol glycosides (SGs), which are about 300 times sweeter than sugar. The present work analyzed the expression of various genes of the SGs biosynthesis pathway in different organs of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service