04473
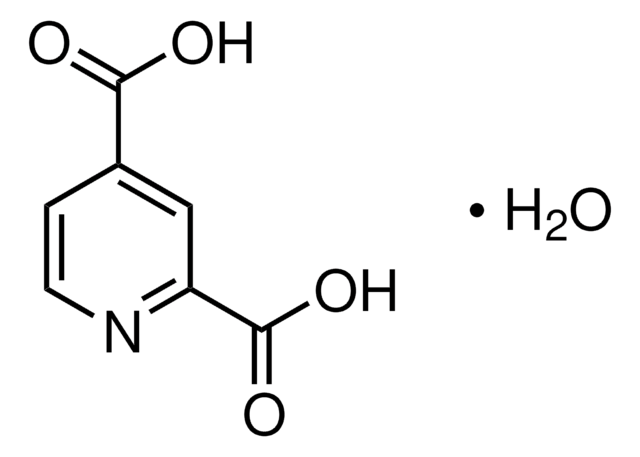
2,4-Pyridinedicarboxylic acid
≥98.0%
Synonym(s):
Lutidinic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H5NO4
CAS Number:
Molecular Weight:
167.12
Beilstein:
131631
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Assay
≥98.0%
98.0-102.0% (T)
SMILES string
OC(=O)c1ccnc(c1)C(O)=O
InChI
1S/C7H5NO4/c9-6(10)4-1-2-8-5(3-4)7(11)12/h1-3H,(H,9,10)(H,11,12)
InChI key
MJIVRKPEXXHNJT-UHFFFAOYSA-N
Application
2,4-Pyridinedicarboxylic acid is an in vitro and in cell inhibitor, as well as a known inhibitor of the histone lysine demethylases. 2,4-Pyridinedicarboxylic acid has been used in a study to determine that ruthenium(II) complexes exert antimetastatic effects on several tumor cell lines in vitro, achieved mostly by the effect on cell adhesion, migration and angiogenesis. . 2,4-Pyridinedicarboxylic acid has been used in a study to develop an assay that represents the first report of a RapidFire mass spectrometery assay for an epigenetics target.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rajarshi Choudhury et al.
FEBS letters, 581(14), 2733-2736 (2007-05-29)
Irrespective of their pyridine nucleotide specificity, all glutamate dehydrogenases share a common chemical mechanism that involves an enzyme bound 'iminoglutarate' intermediate. Three compounds, structurally related to this intermediate, were tested for the inhibition of purified NADP-glutamate dehydrogenases from two Aspergilli
Sue E Hutchinson et al.
Journal of biomolecular screening, 17(1), 39-48 (2011-08-24)
A high-throughput RapidFire mass spectrometry assay is described for the JMJD2 family of Fe(2+), O(2), and α-ketoglutarate-dependent histone lysine demethylases. The assay employs a short amino acid peptide substrate, corresponding to the first 15 amino acid residues of histone H3
G Tschank et al.
The Biochemical journal, 275 ( Pt 2), 469-476 (1991-04-15)
The biochemical and morphological consequences of procollagen prolyl 4-hydroxylase inhibition by pyridine-2,4-dicarboxylic acid (2,4-PDCA) and its diethyl ester (diethyl-2,4-PDC) were studied in chick-embryo calvaria, which predominantly synthesize type I collagen. Half-maximal inhibition of tissue hydroxyproline formation required 650 microM-2,4-PDCA, whereas
Line H Kristensen et al.
The FEBS journal, 279(11), 1905-1914 (2012-03-17)
Dynamic methylations and demethylations of histone lysine residues are important for gene regulation and are facilitated by histone methyltransferases and histone demethylases (HDMs). KDM5B/Jarid1B/PLU1 is an H3K4me3/me2-specific lysine demethylase belonging to the JmjC domain-containing family of histone demethylases (JHDMs). Several
M Kristian Koski et al.
The Journal of biological chemistry, 282(51), 37112-37123 (2007-10-18)
Prolyl 4-hydroxylases (P4Hs) are 2-oxoglutarate dioxygenases that catalyze the hydroxylation of peptidyl prolines. They play an important role in collagen synthesis, oxygen homeostasis, and plant cell wall formation. We describe four structures of a P4H from the green alga Chlamydomonas
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service