217247
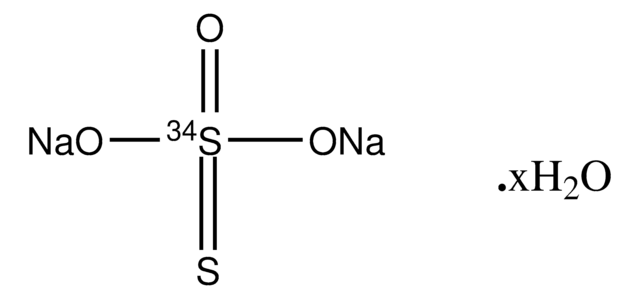
Sodium thiosulfate pentahydrate
ACS reagent, ≥99.5%
About This Item
Recommended Products
grade
ACS reagent
Agency
suitable for EPA 1621
suitable for ISO 25101
suitable for SM 2310
suitable for SM 2320
suitable for SM 4500 - NH3
Assay
≥99.5%
form
crystalline powder
crystals
pellets
impurities
≤0.002% N compounds
≤0.005% insolubles
pH
6.0-8.4 (25 °C, 5%)
solubility
water: soluble(lit.)
anion traces
S2-: passes test
SO42- and SO32-: ≤0.1%
cation traces
N: ≤0.002%
SMILES string
O.O.O.O.O.[Na+].[Na+].[O-]S([O-])(=O)=S
InChI
1S/2Na.H2O3S2.5H2O/c;;1-5(2,3)4;;;;;/h;;(H2,1,2,3,4);5*1H2/q2*+1;;;;;;/p-2
InChI key
PODWXQQNRWNDGD-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Thioester derivatives via one-pot two-step reactions with organic halides and aryl anhydrides.
- Sulfur nanoparticles using F. benghalensis leaf extract which acts as a reducing and capping agent.
- Unsymmetrical heteroaryl thioethers via multicomponent reaction with heteroaryl chlorides and alcohols.
It can also be used in the fabrication of microencapsulated phase change materials for thermal energy storage applications.
Features and Benefits
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service