M49887
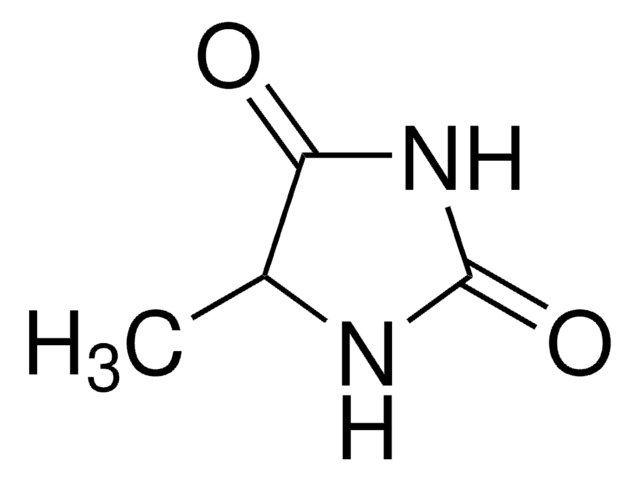
1-Methylhydantoin
97%
Synonym(s):
1-Methylimidazolidine-2,4-dione, Dioxy-creatinine, NSC 80560
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H6N2O2
CAS Number:
Molecular Weight:
114.10
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
156-157 °C (lit.)
SMILES string
CN1CC(=O)NC1=O
InChI
1S/C4H6N2O2/c1-6-2-3(7)5-4(6)8/h2H2,1H3,(H,5,7,8)
InChI key
RHYBFKMFHLPQPH-UHFFFAOYSA-N
Related Categories
Application
Reactant for organocatalytic tandem three component reactions of aldehyde, alkyl vinyl ketone, and amide
Reactant for synthesis of:
Reactant for synthesis of:
- Selective angiotensin II AT2 receptor agonists with reduced CYP 450 inhibition
- Allosteric glucokinase activators
- Hydantoin derivatives with antiproliferative activity
- Thiohydantoins
- P2X7 receptor antagonists
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
K Ienaga et al.
Biochimica et biophysica acta, 967(3), 441-443 (1988-12-15)
The metabolic pathway of 1-methylhydantoin (2) via 5-hydroxy-1-methylhydantoin (3), methylparabanic acid (4) and N5-methyloxaluric acid (5) proved to be a major and general one in mammals. Hence the formation of (3), which has not been detected in normal tissue, is
[1-Methylhydantoin, an unexpected metabolite of the intelligence-affecting substance dupracetam (author's transl)].
H D Dell et al.
Archiv der Pharmazie, 314(8), 697-702 (1981-08-01)
Hybrid biosensor for clinical and fermentation process control.
I Karube et al.
Annals of the New York Academy of Sciences, 434, 508-511 (1984-01-01)
M W Sundberg et al.
Clinical chemistry, 29(4), 645-649 (1983-04-01)
We developed a thin-film enzymic assay for creatinine that makes use of creatinine iminohydrolase (EC 3.5.4.21) to convert creatinine to N-methylhydantoin and ammonia. The ammonia diffuses through a semipermeable layer and is quantitated by reaction with bromphenol blue. A paired
J M Kim et al.
Biochemical and biophysical research communications, 142(3), 1006-1012 (1987-02-13)
A new enzyme, N-methylhydantoin amidohydrolase, was highly purified from Pseudomonas putida 77: it catalyzes the hydrolysis of N-methylhydantoin to N-carbamoylsarcosine with the concomitant stoichiometric cleavage of ATP to ADP and orthophosphate. The enzyme absolutely requires ATP, MG2+ and K+ for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service