D193607
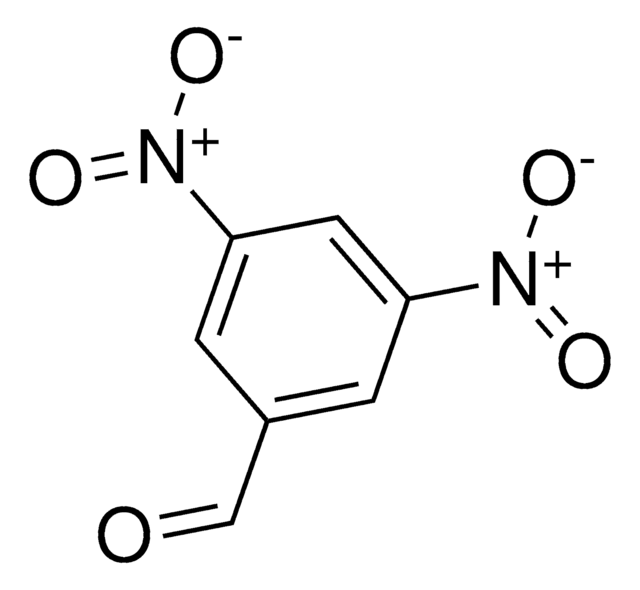
2,4-Dinitrobenzaldehyde
97%
Synonym(s):
2,4-Dinitrobenzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(O2N)2C6H3CHO
CAS Number:
Molecular Weight:
196.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
bp
190 °C/10 mmHg (lit.)
mp
66-70 °C (lit.)
SMILES string
[O-][N+](=O)c1ccc(C=O)c(c1)[N+]([O-])=O
InChI
1S/C7H4N2O5/c10-4-5-1-2-6(8(11)12)3-7(5)9(13)14/h1-4H
InChI key
ZILXIZUBLXVYPI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Sayama et al.
Xenobiotica; the fate of foreign compounds in biological systems, 19(1), 83-92 (1989-01-01)
1. The major biliary metabolite of 2,4-dinitrotoluene (2,4-DNT) in male Wistar rat was 2,4-dinitrobenzyl alcohol glucuronide and the minor metabolites were 2,4-dinitrobenzyl alcohol, 2,4-dinitrobenzaldehyde, 2-acetylamino-4-nitrotoluene, 4-amino-2-nitro(2-amino-4-nitro)benzyl alcohol sulphate, 2,4-dinitrobenzoic acid, 2,4-diacetylaminobenzoic acid and 2-amino-4-nitrobenzoic acid. 2. 2,4-Dinitrobenzyl alcohol, 2,4-dinitrobenzaldehyde, 2,4-dinitrobenzyl
Metabolism of 2,4-dinitrotoluene, 2,4-dinitrobenzyl alcohol and 2,4-dinitrobenzaldehyde by rat liver microsomal and cytosol fractions.
M Shoji et al.
Chemical & pharmaceutical bulletin, 35(4), 1579-1586 (1987-04-01)
M Sayama et al.
Mutation research, 243(1), 47-52 (1990-01-01)
The mutagenicities and theoretical reactivity indices of 2,4-dinitrobenzaldehyde (2,4-DNBAl) and 2,6-dinitrobenzaldehyde (2,6-DNBAl) were investigated using Salmonella typhimurium strains TA98, TA98NR, TA98/1,8-DNP6, and TA100, TA100NR and TA100/1,8-DNP6, by means of the modified intermediate neglect of differential overlap/3 (MINDO)/3) method. The mutagenic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service









![Tris[2-phenylpyridinato-C2,N]iridium(III) 97%](/deepweb/assets/sigmaaldrich/product/structures/167/234/658d0b76-d31d-4fd5-8041-e04e207227c9/640/658d0b76-d31d-4fd5-8041-e04e207227c9.png)