All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H7Br
CAS Number:
Molecular Weight:
183.05
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
refractive index
n20/D 1.59 (lit.)
bp
90 °C/10.5 mmHg (lit.)
density
1.45 g/mL at 25 °C (lit.)
SMILES string
BrC1Cc2ccccc12
InChI
1S/C8H7Br/c9-8-5-6-3-1-2-4-7(6)8/h1-4,8H,5H2
InChI key
AYNXHFRDABNHRX-UHFFFAOYSA-N
Related Categories
General description
1-Bromobenzocyclobutene is a useful synthon. It has important applications in organometallic methodology. Reaction between cycloheptatriene, bromoform, potassium carbonate and 18-crown-6 at 140°C yields 1-bromobenzocyclobutene.
Application
1-Bromobenzocyclobutene may be used in the synthesis of following compounds:
- five-membered zirconacycles
- benzocyclobutenol and benzocyclobutenone
- 2,3-dimethoxyprotoberberinium bromide, via reaction with 3,4-dihydro-6,7-dimethoxyisoquinoline
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
204.8 °F - closed cup
Flash Point(C)
96.00 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
1-Bromobenzocyclobutene: a convenient entry into the benzocyclobutene ring system.
DeCamp MR and Viscogliosi LA.
The Journal of Organic Chemistry, 46(19), 3918-3920 (1981)
T V V Ramakrishna et al.
Organic letters, 5(6), 877-879 (2003-03-14)
[reaction: see text] Commercially available 1-bromobenzocyclobutene is a potentially useful synthon particularly with the application of organometallic methodology. Here we show that it is readily converted into Cp(2)Zr(benzocyclobutadiene), which couples with alkynes or nitriles giving five-membered zirconacycles. Treatment of these
Studies on the syntheses of heterocyclic compounds-DXLV: An alternative synthesis of the protoberberine ring system.
Kametani T, et al.
Tetrahedron, 30(9), 1043-1046 (1974)
Condensed Cyclobutane Aromatic Compounds. IX. Benzocyclobutenol and Benzocyclobutenone.
Cava MP and Muth K.
Journal of the American Chemical Society, 82(3), 652-654 (1960)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service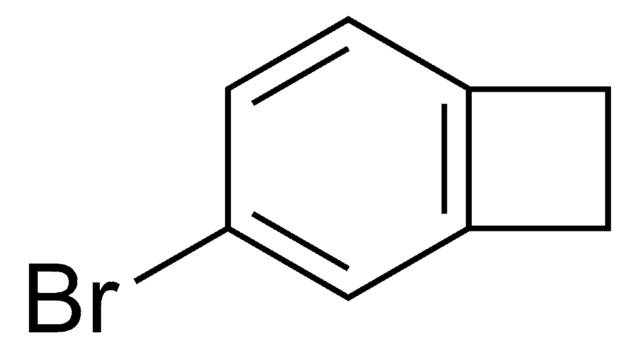
![BICYCLO[4.2.0]OCTA-1,3,5-TRIEN-7-ONE AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/105/021/61bb8f7c-e86a-48bf-8d63-aa68ad3c21df/640/61bb8f7c-e86a-48bf-8d63-aa68ad3c21df.png)