All Photos(1)
About This Item
Empirical Formula (Hill Notation):
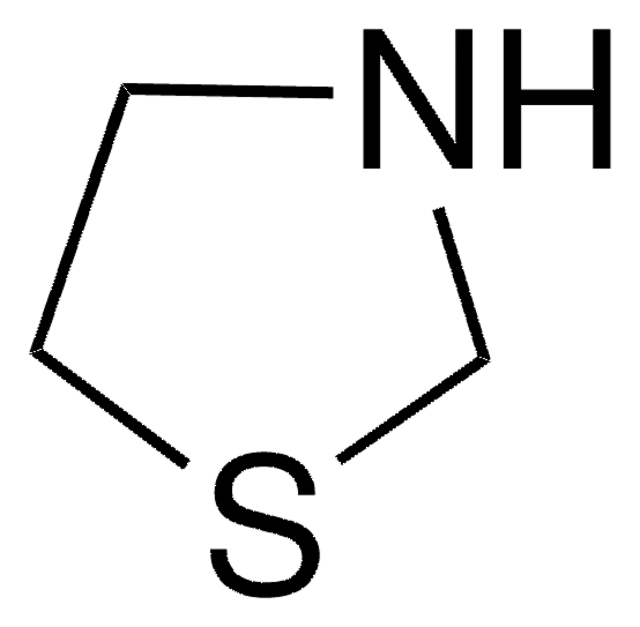
C4H7NO2S
CAS Number:
Molecular Weight:
133.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
176 °C (lit.)
SMILES string
OC(=O)C1NCCS1
InChI
1S/C4H7NO2S/c6-4(7)3-5-1-2-8-3/h3,5H,1-2H2,(H,6,7)
InChI key
ULSZVNJBVJWEJE-UHFFFAOYSA-N
General description
Thiazolidine-2-carboxylic acid (β-thiaproline) is a proline analog. It is an important building block of β-lactam antibiotics. Its X-ray photoelectron spectra has been investigated. It has been reported as a physiological substrate of hog kidney D-amino acid oxidase. Thiazolidine-2-carboxylic acid can be synthesized from cysteamine and glyoxylate.
Application
Thiazolidine-2-carboxylic acid may be used in the synthesis of azabicycloadducts and 5-aryl-2,3-dihydropyrrolo[2,1-b]thiazole-6,7-dimethanol 6,7-bis(isopropylcarbamates).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
V Busiello et al.
Biochimica et biophysica acta, 564(2), 311-321 (1979-09-27)
Thiazolidine-2-carboxylic acid, or beta-thiaproline, is a proline analog in which the beta methylene group of proline is substituted by a sulfur atom. It has been deomonstrated that beta-thiaproline is activated and transferred to tRNAPro by Escherichia coli and rat liver
C L Burns et al.
Biochemical and biophysical research communications, 125(3), 1039-1045 (1984-12-28)
Adducts of glyoxylate with L-cysteine or L-cysteinylglycine were found to be excellent substrates at low concentrations for beef kidney D-aspartate oxidase. Evidence is presented that cis-thiazolidine-2,4-dicarboxylate and its glycine amide are the actual substrates, and that both are converted in
L Włodek et al.
Biochemical pharmacology, 46(1), 190-193 (1993-07-06)
2-Substituted thiazolidine-4(R)-carboxylic acids (TD) were found to increase the concentration of non-protein sulphydryls (NPSH) and the activity of rhodanese (thiosulphate sulphurtransferase, EC 2.8.1.1) and 3-mercaptopyruvate sulphurtransferase (EC 2.8.1.2) in mouse liver. These properties suggest TDs are potentially hepatoprotective compounds. However
Synthesis and chromatographic properties of selenazolidine-2-carboxylic acid (beta-selenaproline).
C De Marco et al.
The Italian journal of biochemistry, 28(2), 104-110 (1979-03-01)
P F Fitzpatrick et al.
The Journal of biological chemistry, 257(3), 1166-1171 (1982-02-10)
A mixture of cysteamine and glyoxylate, proposed by Hamilton et al. to form the physiological substrate of hog kidney D-amino acid oxidase (Hamilton, G. A., Buckthal, D. J., Mortensen, R. M., and Zerby, K. W. (1979) Proc. Natl. Acad. Sci.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![2-[2-(Dimethylamino)ethoxy]ethanol 98%](/deepweb/assets/sigmaaldrich/product/structures/194/219/0fc5e46a-3a2e-48e4-b3b3-c63b710a5f99/640/0fc5e46a-3a2e-48e4-b3b3-c63b710a5f99.png)
![3,7-Diacetyl-1,3,7-triaza-5-phosphabicyclo[3.3.1]nonane 97%](/deepweb/assets/sigmaaldrich/product/structures/198/979/42d0b946-b026-4831-b284-fcb0e91533d9/640/42d0b946-b026-4831-b284-fcb0e91533d9.png)