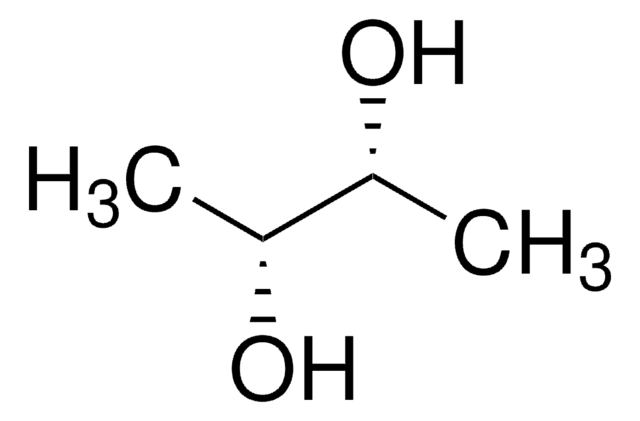
377619
D-Threitol
99%
Synonym(s):
(2R,3R)-1,2,3,4-Butanetetrol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOCH2[CH(OH)]2CH2OH
CAS Number:
Molecular Weight:
122.12
Beilstein:
1719752
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
optical activity
[α]20/D −14°, c = 2 in ethanol
mp
88-90 °C (lit.)
SMILES string
OC[C@@H](O)[C@H](O)CO
InChI
1S/C4H10O4/c5-1-3(7)4(8)2-6/h3-8H,1-2H2/t3-,4-/m1/s1
InChI key
UNXHWFMMPAWVPI-QWWZWVQMSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Amplification of dynamic chiral crown ether complexes during cyclic acetal formation.
Benzion Fuchs et al.
Angewandte Chemie (International ed. in English), 42(35), 4220-4224 (2003-09-23)
A Döss et al.
Physical review letters, 88(9), 095701-095701 (2002-02-28)
We have studied details of the molecular origin of slow secondary relaxation near T(g) in a series of neat polyalcohols by means of dielectric spectroscopy and (2)H NMR. From glycerol to threitol, xylitol, and sorbitol the appearance of the secondary
B J Ortwerth et al.
Experimental eye research, 58(6), 665-674 (1994-06-01)
L-Threose is a significant degradation product of ascorbic acid at pH 7.0 in the presence of oxygen. When compared to several other ascorbate-derived degradation products, it had the greatest ability to glycate and crosslink lens proteins in vitro. To determine
M C Alliegro
Analytical biochemistry, 282(1), 102-106 (2000-06-22)
Dithiothreitol (DTT) is widely used to reduce disulfide bonds in the analysis of protein structure and function. However, thiol-disulfide exchange is not the only mechanism whereby DTT can alter protein function. We observe that DTT diminishes the carbohydrate binding activity
Jonathan D Silk et al.
Journal of immunology (Baltimore, Md. : 1950), 180(10), 6452-6456 (2008-05-06)
Invariant NKT cells (iNKT cells) recognize CD1d/glycolipid complexes. We demonstrate that the nonglycosidic compound threitolceramide efficiently activates iNKT cells, resulting in dendritic cell (DC) maturation and the priming of Ag-specific T and B cells. Threitolceramide-pulsed DCs are more resistant to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service