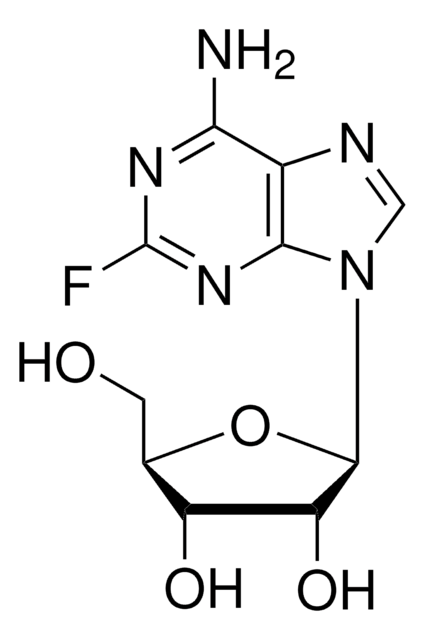
T3580
Toyocamycin
≥98% (HPLC), from Streptomyces rimosus
Sinónimos:
4-Aminopyrrolo[2,3-d]pyrimidine-5-carbonitrile 7-(β-D-ribofuranoside), 7-Deaza-7-cyanoadenosine, NSC 63701, NSC 99843, Neuro 000027, Unamycin B, Vengicide
About This Item
Productos recomendados
biological source
Streptomyces rimosus
Quality Level
assay
≥98% (HPLC)
form
solid
solubility
DMSO: soluble 0.90-1.10 mg/mL, clear, colorless
H2O: moderately soluble
aqueous acid: moderately soluble
ethanol: moderately soluble
methanol: moderately soluble
antibiotic activity spectrum
fungi
mode of action
DNA synthesis | interferes
Storage temp.
2-8°C
SMILES string
Nc1ncnc2n(cc(C#N)c12)[C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O
InChI
1S/C12H13N5O4/c13-1-5-2-17(11-7(5)10(14)15-4-16-11)12-9(20)8(19)6(3-18)21-12/h2,4,6,8-9,12,18-20H,3H2,(H2,14,15,16)/t6-,8-,9-,12-/m1/s1
Inchi Key
XOKJUSAYZUAMGJ-WOUKDFQISA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Biochem/physiol Actions
Features and Benefits
Preparation Note
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
We offers many products related to adenosine receptors for your research needs.
We offers many products related to adenosine receptors for your research needs.
We offers many products related to adenosine receptors for your research needs.
We offers many products related to adenosine receptors for your research needs.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico









![PERK Inhibitor I, GSK2606414 GSK2606414 is a cell-permeable, highly potent inhibitor of EIF2AK3/PERK (IC₅₀ = 0.4 nM; [ATP] = 5 µM). Targets PERK in its inactive DFG conformation at the ATP-binding region.](/deepweb/assets/sigmaaldrich/product/structures/180/559/efa716dc-d5fe-4339-a6f0-0103084fc04a/640/efa716dc-d5fe-4339-a6f0-0103084fc04a.png)