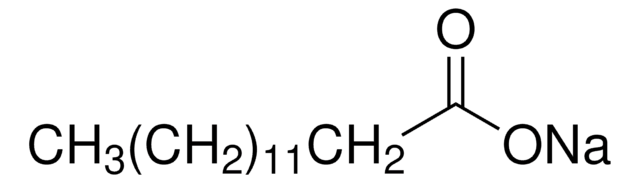W276405
Myristic acid
≥95%, FCC, FG
Sinónimos:
1-Tridecanecarboxylic acid, C14:0, NSC 5028, Tetradecanoic acid
About This Item
Productos recomendados
biological source
synthetic
Quality Level
grade
FG
Fragrance grade
Halal
Kosher
agency
follows IFRA guidelines
meets purity specifications of JECFA
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 172.210
FDA 21 CFR 172.860
FDA 21 CFR 173.340
assay
≥95%
bp
250 °C/100 mmHg (lit.)
mp
52-54 °C (lit.)
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
organoleptic
oily; waxy; soapy
SMILES string
CCCCCCCCCCCCCC(O)=O
InChI
1S/C14H28O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14(15)16/h2-13H2,1H3,(H,15,16)
InChI key
TUNFSRHWOTWDNC-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- Experiments and Molecular Simulations to Study the Effect of Surface-Active Compounds in Mixtures of Model Oils on CO2 Corrosion during Intermittent Oil-Water Wetting.: Investigates how myristic acid, as a surface-active compound, influences CO2 corrosion mechanisms in oil-water systems, offering insights into corrosion prevention strategies in the oil industry (Norooziasl et al., 2024).
Disclaimer
Storage Class
11 - Combustible Solids
wgk_germany
nwg
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico