776246
SPhos Pd G3
97%
Sinónimos:
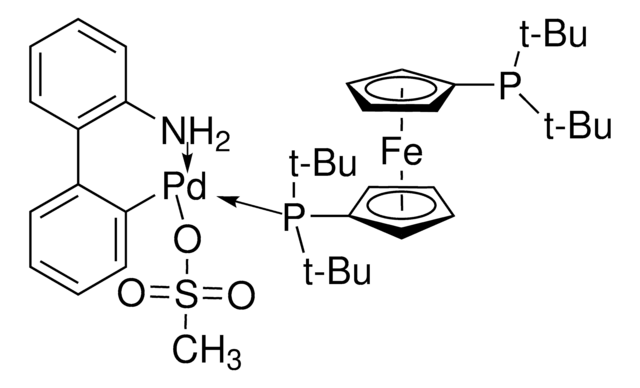
(2-Dicyclohexylphosphino-2′,6′-dimethoxybiphenyl) [2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate
About This Item
Productos recomendados
Nivel de calidad
Ensayo
97%
Formulario
solid
Características
generation 3
idoneidad de la reacción
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
197-214 °C
grupo funcional
phosphine
temp. de almacenamiento
2-8°C
cadena SMILES
CS(=O)(=O)O[Pd]c1ccccc1-c2ccccc2N.COc3cccc(OC)c3-c4ccccc4P(C5CCCCC5)C6CCCCC6
InChI
1S/C26H35O2P.C12H10N.CH4O3S.Pd/c1-27-23-17-11-18-24(28-2)26(23)22-16-9-10-19-25(22)29(20-12-5-3-6-13-20)21-14-7-4-8-15-21;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h9-11,16-21H,3-8,12-15H2,1-2H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1
Clave InChI
SCWODMZBSVVMRH-UHFFFAOYSA-M
Descripción general
Aplicación
Producto relacionado
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
Contenido relacionado
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)


![[1,1′-bis(difenilfosfino)ferroceno]dicloropaladio(II), complejo con diclorometano](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)