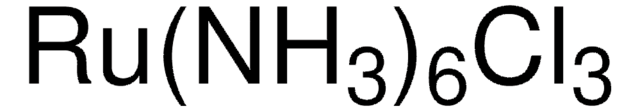450162
Potassium hexachloroiridate(IV)
99.99% trace metals basis
Sinónimos:
Iridium potassium chloride
About This Item
Productos recomendados
Quality Level
assay
99.99% trace metals basis
form
powder
impurities
≤150.0 ppm Trace Metal Analysis
mp
>385 °C (lit.)
SMILES string
[K+].[K+].Cl[Ir--](Cl)(Cl)(Cl)(Cl)Cl
InChI
1S/6ClH.Ir.2K/h6*1H;;;/q;;;;;;+4;2*+1/p-6
InChI key
WEKZJZDULNNSCD-UHFFFAOYSA-H
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- Voltammetry at HMT-PMBI-coated glassy carbon electrodes: This study explores the electrochemical properties of potassium hexachloroiridate(IV) and its potential for sensitive detection, which could be valuable in analytical chemistry applications (M Rees et al., 2020).
- Antioxidant Total Capacity Assay: Introduces a novel assaying method for antioxidant capacity using potassium hexachloroiridate(IV), which could have implications in pharmacology and drug discovery (Y Liu et al., 2024).
- Mass Transfer in 3D-printed Electrolyzers: Examines the role of potassium hexachloroiridate(IV) in enhancing mass transfer in electrolytic systems, which is pivotal for chemical engineering and energy applications (SJC Weusten et al., 2021).
signalword
Warning
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico