405817
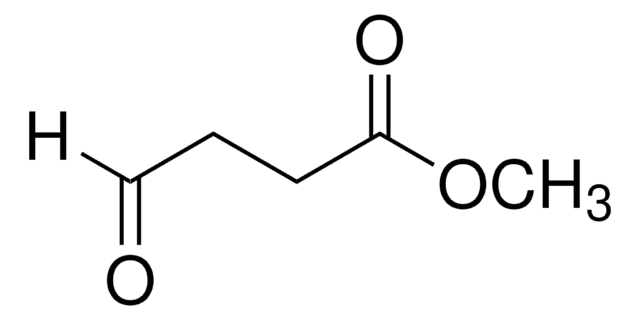
Methyl 5,5-dimethoxyvalerate
96%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
(CH3O)2CH(CH2)3CO2CH3
Número de CAS:
Peso molecular:
176.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
96%
form
liquid
refractive index
n20/D 1.422 (lit.)
bp
70-72 °C/2 mmHg (lit.)
density
1.012 g/mL at 25 °C (lit.)
functional group
acetal
ester
ether
SMILES string
COC(CCCC(=O)OC)OC
InChI
1S/C8H16O4/c1-10-7(9)5-4-6-8(11-2)12-3/h8H,4-6H2,1-3H3
InChI key
YOFAONQHOIRLCQ-UHFFFAOYSA-N
Categorías relacionadas
General description
Methyl 5,5-dimethoxyvalerate (methyl 5,5-dimethoxypentanoate) is an ester. It can be prepared by reacting methyl 5-oxopentanoate with p-toluene sulfonic acid and trimethylorthoformate. It participates in the synthesis of 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphatidylcholine.
Application
Methyl 5,5-dimethoxyvalerate may be employed in the synthesis of seven-membered carbocycles. It may be used in the synthesis of 5-(phenylamino)-4-(phenylimino)methyl)-4-pentenoic acid derivatives.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
145.4 °F - closed cup
flash_point_c
63 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Sanjay Srivastava et al.
The Journal of biological chemistry, 279(51), 53395-53406 (2004-10-07)
Oxidation of unsaturated phospholipids results in the generation of aldehyde side chains that remain esterified to the phospholipid backbone. Such "core" aldehydes elicit immune responses and promote inflammation. However, the biochemical mechanisms by which phospholipid aldehydes are metabolized or detoxified
Fangwei Shao et al.
Bioconjugate chemistry, 19(12), 2487-2491 (2008-12-05)
A facile synthetic route to prepare monofunctional carbocyanine dyes for biological application is developed. Three pentamethine carbocyanine dyes have been successfully modified with a variety of functional groups such as: carboxylic acids, azides, or alkynes. The new dyes are characterized
Formation of seven-membered carbocycles by the use of cyclopropyl silyl ethers as homoenols.
Oleg L Epstein et al.
Angewandte Chemie (International ed. in English), 45(30), 4988-4991 (2006-07-05)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico