345164
Tin(II) acetate
Sinónimos:
Stannous acetate, Tin acetate, Tin diacetate
About This Item
Productos recomendados
form
solid
reaction suitability
core: tin
reagent type: catalyst
mp
180-182 °C (lit.)
SMILES string
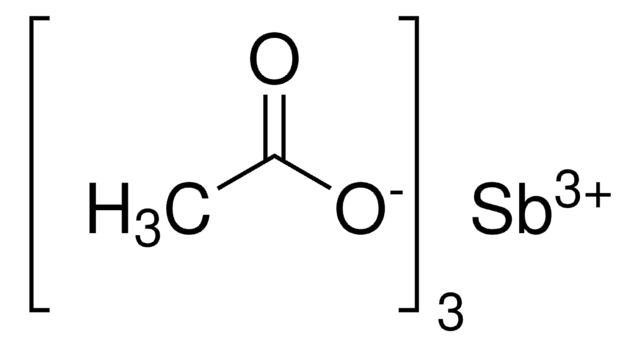
CC(=O)O[SnH2]OC(C)=O
InChI
1S/2C2H4O2.Sn/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
InChI key
PNOXNTGLSKTMQO-UHFFFAOYSA-L
Categorías relacionadas
General description
Application
- Enhances the rate of thermal depolymerization of poly(lactic acid) fibers
- Reactant for the synthesis of Sn-Cu bimetallic nanoparticles
- used in in preparation of tin anode by organic electroplating for rechargeable thin-film batteries
- Used as tin source for preparation of high surface area tin oxide catalysts
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico