296783
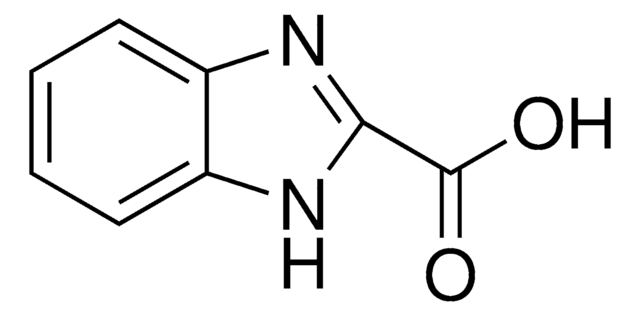
5-Benzimidazolecarboxylic acid
96%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C8H6N2O2
Número de CAS:
Peso molecular:
162.15
Número MDL:
Código UNSPSC:
12352100
eCl@ss:
32151902
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Ensayo
96%
mp
>300 °C (lit.)
grupo funcional
carboxylic acid
cadena SMILES
OC(=O)c1ccc2[nH]cnc2c1
InChI
1S/C8H6N2O2/c11-8(12)5-1-2-6-7(3-5)10-4-9-6/h1-4H,(H,9,10)(H,11,12)
Clave InChI
COYPLDIXZODDDL-UHFFFAOYSA-N
Descripción general
Drug-specific monoclonal antibodies were produced against the very small drug hapten, 5-benzimidazolecarboxylic acid.
Aplicación
5-Benzimidazolecarboxylic acid has been used in the preparation of:
- 1H-benzoimidazole-5-carboxylic acid benzotriazol-1-yl ester
- piperidin-1-yl(1-m-tolyl-1H-benzo[d]imidazol-5-yl)methanone
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
E S Medlock et al.
The Anatomical record, 207(1), 31-41 (1983-09-01)
Mouse fetal liver was studied ultrastructurally to identify and characterize the developing hepatic parenchyma or prehepatocyte which may be responsible for producing the liver hemopoietic environment. It was observed that as the liver develops, there is close association of endodermal
Ajit Jadhav et al.
Probe Reports from the NIH Molecular Libraries Program, 2010 Mar 4 (Updated 2011 Mar 11) (2011-07-08)
15-hydroxyprostaglandin dehydrogenase (15-PGDH; HPGD) is the key enzyme for the inactivation of prostaglandins, and thus regulates processes such as inflammation or proliferation. The anabolic pathways of prostaglandins are well-characterized, especially with respect to regulation of the cyclooxygenase (COX) enzymes. In
E Moran et al.
Journal of immunological methods, 271(1-2), 65-75 (2002-11-26)
Drug-specific monoclonal antibodies (MAbs) were produced against the very small drug hapten (162.15 Da), 5-benzimidazolecarboxylic acid, an analogue of 2-(4-Thiazolyl)benzimidazole (TBZ) but lacking the thiol group. TBZ is widely used as a broad-spectrum anthelmintic in various animal species and humans
Xiaofei Chen et al.
Biomaterials science, 3(6), 870-878 (2015-07-30)
Herein, hyperbranched poly(ethylene glycol)-based supramolecular nanoparticles with pH-sensitive properties were designed and used for targeted drug delivery. Via host-guest recognition between benzimidazole anchored poly(ethylene glycol)-hyperbranched polyglycerol (PEG-HPG-BM) and folic acid modified CD (FA-CD), targeted supramolecular nanoparticles (TSNs) were fabricated. At
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico