185922
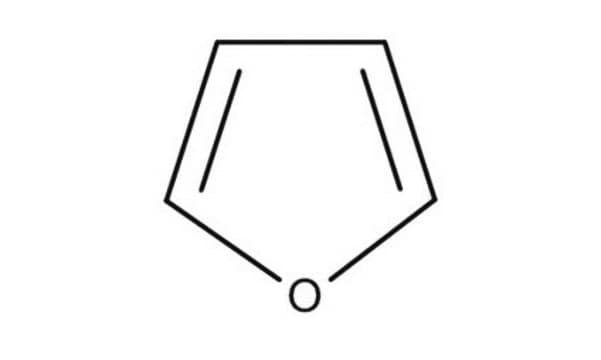
Furan
≥99%
Sinónimos:
1,4-Epoxybuta-1,3-diene, Divinylene oxide, Oxacyclopentadiene, Oxole, Tetrole
About This Item
Productos recomendados
vapor density
2.35 (vs air)
Quality Level
vapor pressure
1672 mmHg ( 55 °C)
31.66 psi ( 55 °C)
493 mmHg ( 20 °C)
9.22 psi ( 20 °C)
assay
≥99%
form
liquid
contains
0.025 wt. % BHT as inhibitor
expl. lim.
14.3 %
refractive index
n20/D 1.421 (lit.)
bp
32 °C/758 mmHg (lit.)
solubility
alcohols: freely soluble
diethyl ether: freely soluble
water: insoluble
density
0.936 g/mL at 25 °C (lit.)
shipped in
wet ice
storage temp.
2-8°C
SMILES string
c1ccoc1
InChI
1S/C4H4O/c1-2-4-5-3-1/h1-4H
InChI key
YLQBMQCUIZJEEH-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- Preparation of the starting material required for the synthesis of calix[6]pyrrole.
- To investigate the kinetics and mechanism of reactions of chlorine atoms with volatile organic compounds.
- Catalytic transformation of furan to aromatics and olefins.
signalword
Danger
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 1B - Flam. Liq. 1 - Muta. 2 - Skin Irrit. 2 - STOT RE 2
supp_hazards
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
-32.8 °F - closed cup
flash_point_c
-36 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico