167134
Bromohydroquinone
97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
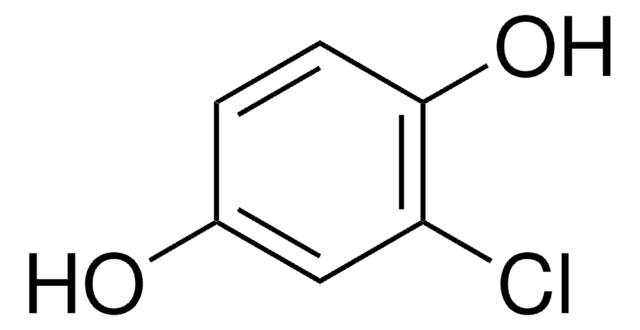
BrC6H3(OH)2
Número de CAS:
Peso molecular:
189.01
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
97%
mp
112-116 °C (lit.)
functional group
bromo
SMILES string
Oc1ccc(O)c(Br)c1
InChI
1S/C6H5BrO2/c7-5-3-4(8)1-2-6(5)9/h1-3,8-9H
InChI key
REFDOIWRJDGBHY-UHFFFAOYSA-N
Categorías relacionadas
Application
Bromohydroquinone was used in the synthesis of Π-conjugated polymers composed of alkyl carbazole/dialkoxyphenylene and squaraine units via Sonogashira cross-coupling reactions. It was used in the preparation of 2-bromobenzoquinone.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
X Yang et al.
Journal of toxicology and environmental health, 48(4), 319-332 (1996-07-01)
The role of proteinases in renal proximal tubule (RPT) cellular death was examined using specific inhibitors of proteinases. Rabbit RPT suspensions were incubated with antimycin A for 1 h or tetrafluoroethyl-L-cysteine (TFEC) for 4 h in the absence or presence
R G Schnellmann et al.
Toxicology and applied pharmacology, 90(3), 420-426 (1987-09-30)
2-Bromohydroquinone (BHQ) is a nephrotoxic metabolite of bromobenzene and a model toxic hydroquinone. The primary goal of these studies was to determine whether BHQ produces toxicity in rabbit renal proximal tubules by inhibiting mitochondrial function. BHQ induces a specific sequence
J E Andrews et al.
Toxicology and applied pharmacology, 120(1), 1-7 (1993-05-01)
Glutathione conjugates of 2-bromohydroquinone (GSyl-BHQ) cause renal proximal tubular necrosis that is dependent upon the activity of gamma-glutamyl transferase (GGT). GGT is present in embryonic yolk sac and its activity increases with gestational age, suggesting that the developing embryo might
T J Monks et al.
Molecular pharmacology, 34(1), 15-22 (1988-07-01)
The formation of potentially reactive thiols has been postulated to play a role in the nephrotoxicity caused by a number of glutathione and/or cysteine conjugates. However, the inherent reactivity of such compounds has precluded both their identification in biological systems
Synthesis and characterization of novel poly (aryleneethynylene) s derived from squaraines for photovoltaic applications.
Zhang W, et al.
J. Mater. Sci., 46(16), 5363-5370 (2011)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico