111279
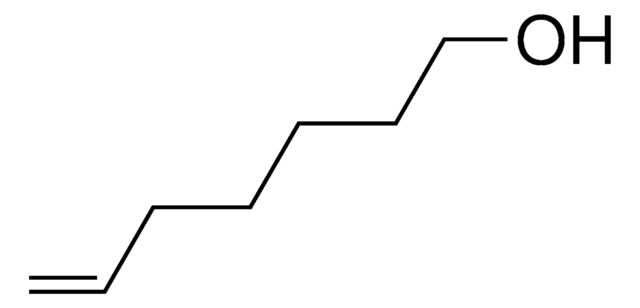
4-Penten-1-ol
99%
Sinónimos:
2-Allylethyl alcohol
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH2=CH(CH2)3OH
Número de CAS:
Peso molecular:
86.13
Beilstein/REAXYS Number:
1560163
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39020310
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
99%
refractive index
n20/D 1.429 (lit.)
bp
134-137 °C (lit.)
density
0.834 g/mL at 25 °C (lit.)
SMILES string
OCCCC=C
InChI
1S/C5H10O/c1-2-3-4-5-6/h2,6H,1,3-5H2
InChI key
LQAVWYMTUMSFBE-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
4-penten-1-ol forms ester bond at the C terminus of the linear peptide in solution with HATU as coupling agent.
Application
4-Penten-1-ol can be used as a reactant to prepare sulfamate ester by reacting with chlorosulfonyl isocycanate (142662).The derived ester undergoes an enantioselective intramolecular azridination reaction in the presence of Cu catalyst. 4-Penten-1-ol can also be used to study the epoxidation of olefins with oxo-diperoxo tungstate(VI) complex as catalyst and bicarbonate as co-catalyst.
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Enantioselective Intramolecular Copper-Catalyzed Aziridination of Sulfamates
Audrey Esteoule.et al.
Synthesis, 1251-1251 (2007)
Highly efficient epoxidation method of olefins with hydrogen peroxide as terminal oxidant, bicarbonate as a co-catalyst and oxodiperoxo molybdenum(VI) complex as catalyst.
Maiti SK, et al.
New. J. Chem., 30(3), 479-489 (2006)
Marina D Rvovic et al.
Journal of molecular modeling, 17(6), 1251-1257 (2010-08-17)
The mechanism of phenylselenoetherification of pent-4-en-1-ol using some bases (pyridine, triethylamine, quinoline, 2,2'-bipyridine) as catalyst was examined through studies of kinetics of the cyclization, by UV-VIS spectrophotometry. It was demonstrated that the intramolecular cyclization is facilitated in the presence of
Stefania Terracciano et al.
Bioorganic & medicinal chemistry, 16(13), 6580-6588 (2008-05-30)
In the recent years, we focused our attention on the cyclodepsipeptide Jaspamide 1, an interesting marine metabolite, possessing a potent inhibitory activity against breast and prostate cancer, as a consequence of its ability to disrupt actin cytoskeleton dynamics. Although its
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico