91582
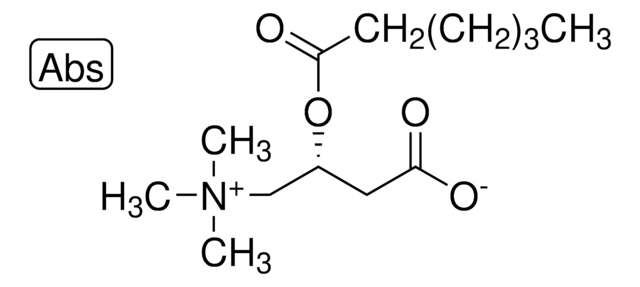
Myristoyl-L-carnitine
analytical standard
Synonym(s):
(2R)-3-Carboxy-N,N,N-trimethyl-2-[(1-oxotetradecyl)oxy]-1-propanaminium inner salt, L-Carnitine tetradecanoyl ester, C14-Carnitine, Tetradecanoyl-L-carnitine
About This Item
Recommended Products
grade
analytical standard
Quality Level
Assay
≥97.0% (HPLC)
optical activity
[α]/D -18.0±2.0°, c = 1 in methanol
shelf life
limited shelf life, expiry date on the label
impurities
≤10.0% water
application(s)
clinical testing
format
neat
storage temp.
2-8°C
SMILES string
C[N+](C)(C)C[C@H](OC(CCCCCCCCCCCCC)=O)CC([O-])=O
InChI
1S/C21H41NO4/c1-5-6-7-8-9-10-11-12-13-14-15-16-21(25)26-19(17-20(23)24)18-22(2,3)4/h19H,5-18H2,1-4H3/t19-/m1/s1
InChI key
PSHXNVGSVNEJBD-LJQANCHMSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
It may be used as an analytical reference standard in the following:
- Separation and identification of underivatized myristoyl-L-carnitine in human plasma samples using high-performance liquid chromatography coupled to tandem mass spectrometry (HPLC-MS/MS).
- Determination of the analyte in dried blood samples using high-performance liquid chromatography (HPLC) coupled with electrospray ionization-tandem mass spectrometry (ESI-MS/MS).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service